. Introduction
The most significant and susceptible phases of a crop cycle are seed germination and seedling emergence, as they require water, air, temperature, and light (Biswas et al., 2019). To obtain the amino acids and energy necessary for plant growth, a complex metabolic process oxidises the carbohydrates and lipids within the seed and separates protein molecules (Almansouri et al., 2001). Low quality of seed and planting conditions affect crop productivity, as it influences seed germination and seedling development (Lamichhane et al., 2019). Poor germination and seedling establishment, which have an adverse effect on crop plant growth and development, are caused in part by soil salinity and are accountable for low agricultural productivity (Kooshkaki et al., 2023). In soils containing excessive sodium chloride, plant accessible water is limited, leading to partial hydration of the cell cytoplasm. The metabolism of cells and the activities of macromolecules are exaggerated by such plasmolysis, eventually leading to the termination of growth (Le Rudulier, 2005). Salt impacts seed germination by osmotic effects, ion toxicity, or a combination of the two. Salts absorb and store moisture to the point where it is no longer readily accessible in the soil and, eventually, the osmotic pressure of the soil solution rises. Plants absorb salt from the soil through transporters, which causes ion toxicity and disrupts mineral uptake and ion homeostasis. Salinity causes significant ion accumulation (Na+ and Cl) and inhibits K+ and Ca2+ uptake, resulting in ionic imbalance (Isayenkov & Maathuis, 2019). Salinity can reduce the germination rate and final germination percentage (FG%), resulting in irregular growth and poor yields (Tsegay & Gebreslassie, 2014).
Mineral fertilizers are used by farmers in high production systems to boost crop output and reduce abiotic stress damage (nutrient deficiency, drought, and salinity). Excess fertilizers can negatively impact plant growth and nutritional value and contribute to soil deterioration (Li et al., 2018). The most extensively used fertilizers are nitrogen-based, and they are to blame for the huge nitrogen footprint in agriculture (Hessini et al., 2019). The roots of plants absorb both inorganic (nitrate and ammonium) and organic (urea, amino acids, peptides) forms of nitrogen, i.e. the most prevalent mineral element in plants. In the most natural and agricultural environments, ammonium is the main inorganic source of nitrogen for plants (Liu & von Wirén, 2017). Many halophytes, including Salicornia bigelovii (Kudo & Fujiyama, 2010), Phragmites australis, Spartina alterniflora, and Glyceria maxima (Munzarova et al., 2006), have been found to benefit from ammonium in low N and high NaCl conditions (Hessini et al., 2009).
Seed priming is a novel approach in plant stress physiology that increases plant tolerance to various biotic and abiotic adverse environments (Sheteiwy et al., 2017). A physiological strategy called “Seed priming” accelerates and synchronises seed germination (Wiszniewska, 2021). Controlled hydration treatments in aerated and diluted salt solutions have proven to benefit a wide range of species (Rehman et al., 1998). Recently, various commercial fertilizers (macro- and micro-nutrients) have been used as nutripriming agents to improve the crop yield. For example, nutripriming with ZnSO4 stimulated the development of young seedlings, increased seedling vigour, and increased Zn and B content in grain cowpea (Raj et al., 2020). NH4+ feeding promoted the development of S. alterniflora in high salinity environments by increasing the activity of the key antioxidant enzymes (Hessini et al., 2013). NH4NO3 nutripriming boosted the pre-germinative metabolism, emergence, and early development of rice seedlings. The most robust germinative efficacy was observed in seeds treated with 6 mM NH4NO3, whereas 2 mM NH4NO3 provided greater root length and N–NH4+ content in roots and stimulated nitrogen metabolism in rice seedlings, which resulted in seedlings with superior growth and higher nitrogen content in their tissues (Pereira et al., 2022).
Tomato (Solanum lycopersicum L.) is the world’s second most valuable fruit or vegetable crop from the Solanaceae family. The presence of various health-promoting chemicals, such as carotenoids, vitamins, and phenolic compounds, contribute significantly to their nutritional value (Li et al., 2018). Water shortages, soil salinization, and other abiotic factors all pose challenges to tomato production around the world (Zhou et al., 2019). Tomato seeds are extremely sensitive to saline stress, which can impact their germination and vigour (Giannakoula & Ilias, 2013). Salinity stress causes tomato plants to grow shorter and have a reduced leaf area. The most important indicators for tomato producers are fruit size and total yield per plant, and in saline conditions, leaf relative chlorophyll content, which indicates the amount of nitrogen, a vital factor for photosynthetic improvement, may also be lowered (Zhang et al., 2017). The aim of this study is to examine where NH4+ nutrition enhances the salt tolerance of tomato plants through a priming effect. We investigated the effects of NH4NO3 and (NH4)2SO4 as NH4+ priming agents on seed germination, growth parameters, and chlorophyll, proline, total protein, total phenolic, and total flavonoid content in non-saline and saline environmental conditions.
. Material and methods
Plant material and NH4+ priming treatment
Tomato seeds (Solanum lycopersicum L.) of the local variety NEMA were obtained from the plant protection department, Karachi, and the research was conducted in 2017–2020 at the PCSIR labs complex in Karachi, Pakistan, in collaboration with the Institute of Biotechnology and Genetic Engineering, University of Sindh, Jamshoro, Pakistan. The tomato seeds were surface-sterilized for 5 min with a 5% (w/v) NaClO solution. The disinfectant was then removed by rinsing three to four times with distilled water. The seeds were then placed on tissue paper to dry. For priming, the tomato seeds were immersed in 50 and 100 mmol concentrations of NH4NO3 and 50 and 100 mmol concentrations of (NH4)2SO4, respectively. The seeds were primed in the absence of light for 12 and 24 h at 25 ± 1 °C, while dry and unprimed seeds were used as a control (Table 1). After conditioning, the seeds were thoroughly rinsed three times with distilled water and dried between two layers of tissue paper.
Table 1
Priming agent name with concentration and soaking time.
Experimental design and conditions
The research was conducted in a laboratory and a greenhouse experiment, and eight treatments were designed. The detailed design of the experiment is shown in Table 1. In the lab trial, twenty seeds were taken for each treatment, along with five replica plates and a control, and placed on four layers of filter paper. For tomato seed germination, all Petri plates moistened with 25 mL of distilled water were incubated at 25 °C. The germinated seedlings were counted every 24 h for 15 days (Association, 1985). The following parameters were assessed in this experiment:
The germination percentage (GP) was calculated on the 15th-day post sowing using the formula:
The mean germination time (MGT) in days was calculated according to the equation (Ellis & Roberts, 1981):
n = number of germinated seeds on day, and D = days counted from the beginning of germination.
The germination rate (GR) provides a measure of the time course of seed germination. GR was calculated based on the equation created by Ellis and Roberts (1981). The germination rate is calculated by the formula:
N = germinated seed number at time t and T is days of experiment.
The second pot experiment was conducted in a greenhouse at the tissue culture laboratory, PCSIR labs complex in Karachi, Pakistan, to evaluate the impact of NH4+ priming (NH4NO3 and (NH4)2SO4) on growth parameters and biochemistry of tomato plants in saline and non-saline environments (Table 2). The soil used in the experiment was evaluated before and after the tomato seedlings were planted (Table 3). Tomato seeds were planted in pots with five replicates and treated with tap water after being primed with NH4NO3 and (NH4)2SO4. The tomato seedlings were replanted in garden soil 40 days after sowing, according to RCBD.
Table 2
Effects of NH4+ priming on germination of tomato cv. Nema.
Table 3
Soil analysis before and after harvest of the plants.
| Before Seedling | Control | Primed | Primed Saline | |
|---|---|---|---|---|
| Texture sandy loam | ||||
| pH | 7.1 | 7.12 | 7.01 | 7.19 |
| Electrical conductivity (EC) (µs/cm) | 700 | 712 | 1690 | 9870 |
| Sodium (ppm*) | 8 | 5 | 18 | 15 |
| Potassium (ppm*) | 25 | 13 | 11 | 14 |
| Calcium (ppm*) | 230 | 240 | 560 | 300 |
| Magnesium (ppm*) | 972 | 972 | 3402 | 430 |
| Sulphate (ppm*) | 1.57 | 1.646 | 0.962 | 1.25 |
| Chloride (ppm*) | 1704 | 1065 | 852 | 294 |
Salinity treatments
The seedlings were divided into three groups after transplantation: control (unprimed) watered with regular tap water, primed seeds irrigated with regular tap water, and primed seeds irrigated with increasing concentrations of saltwater. The first application of saltwater (50 mmol) to the soil occurred 40 days after the seedling were transplanted. After a 10-day break, the concentration of saline water (50, 100, 150, and 200 mmol NaCl) was gradually raised.
Morphological variables
After seedlings were transplanted into new pots, the morphological characteristics were observed every seven days. All plants were measured for morphological characteristics such as number of leaves, plant height (cm), and leaf area. The leaf area in cm2 was calculated using a leaf area machine (C1-2O2-Area Meter USA). After the plant was harvested, the fresh and dry weight of the shoot and root was calculated. The fresh weight of the shoot and root was assessed using an Electronic Weighing Balance (g), followed by 72 h of drying in an oven at 72 °C, and finally, the dry weight of the shoot and root was determined.
Physiological variables
The leaf chlorophyll content was measured by the acetone extraction process. Approximately 0.1 g of leaf tissue was homogenized in 10 mL of 95% (v/v) acetone mixture, and tubes were incubated for 48 h at 4 °C. The concentrations of chlorophyll a, b, and carotenes (g/g FW) were determined by measuring the optical density of the extracted sample at 662, 644, and 470 nm, respectively, in new test tubes with replicates for each sample. The chlorophyll content was calculated using the following equation:
Determination of proline, total protein, total phenolic, and flavonoid contents
The contents of proline and total protein in tomato leaves from non-saline and saline conditions were estimated according to the detailed methodologies for proline (Bates et al., 1973) and total protein (Bradford, 1976), as shown in Table S1.
For the total phenolic assay, 0.1 g of leaf tissue was homogenized with 2 mL of absolute ethanol solution using a pestle and mortar. After homogenization, the plant mixture was centrifuged to collect the upper layer of each sample in a test tube. The reaction was carried out with 0.1 mL of supernatant, followed by 1.0 mL of Folin-Ciocalteu reagent and 0.3 mL NaCO3 in the tube. For 30 min, the mixture was kept at room temperature after measuring the optical density at 756 nm against a blank. Gallic acid was used as standard phenolic acid to prepare a standard curve to quantify the total phenolic concentration (Singleton & Rossi, 1965). The total flavonoid content was measured using the methodology proposed by Kim et al. (2003). In a test tube, 0.1 mL of the sample was mixed with 0.3 mL of 5% NaNO2 for the reaction. The reaction mixture was incubated at room temperature for 5 min. The samples were then treated with 0.3 mL of AlCl3 for 5 min at room temperature. The volume of the reaction mixture was increased to 10.0 mL with distilled water by adding 2.0 mL of 1 mol NaOH. The intensity of the product was measured at 510 nm against a rutin standard to assess the flavonoid concentration.
Statistical analysis
All experiments were carried out five times, and the results were statistically analysed with ANOVA (SPSS 18.0; SPSS Inc., Chicago, USA). Using SPSS 18.0, Duncan’s multiple range test was done to determine whether there were significant changes between the treatments. At p < 0.05, different letters indicate significant differences.
. Results
Effects of NH4+ priming on germination
As shown in Table 2, the highest percentage of germination was obtained in treatment T5 (50 mmol (NH4)2SO4, 12 h), followed by T1 and T3 (50 mmol NH4NO3, 12 h & 100 mmol (NH4)2SO4, 12 h), both of which showed 80% germination. The lowest GP among all the treatments were recorded in T7 & T8 at 72%. The germination percentage increased by 51% in all primed treatments, compared to the control. The T7 (100 mmol (NH4)2SO3, 12 h) treatment gave the lowest value of MGT, followed by T5 (50 mmol (NH4)2SO3, 12 h). The MGT of the tomato seeds was reduced in all primed treatments, compared to the control.
Soil analysis
In the field trials, the treated seeds were planted in soil inside a greenhouse and irrigated with two different methods: tap water and saltwater. Table 3 shows the results of a soil analysis performed prior to seed sowing and after plant harvesting.
Effect of NH4+ priming on growth parameters
Plant height (cm)
The effect of the NH4+ priming on seeds grown in the control conditions resulted in a favourable response of plant height, as shown in Table 4. The highest plant height was observed in treatment T6 (81.5 cm), followed by T3 (77.6 cm), compared to NC (45.8 cm), while the lowest plant height was noted in T7 (57.2 cm) among all the treatments. Overall, the primed treatment increased plant height by 77% more than in the Nema control. Using the various chemicals as an NH4+ priming agent resulted in a significant stimulatory effect on plant height in the Nema cultivar in the saline conditions. When compared to the control (unprimed seeds), the uppermost plant height was noted in treatment T2 (67 cm), followed by treatment T1 (66.6 cm), and all the primed treatments yielded the lowest plant heights (T3 and T7), as shown in Table 4.
Table 4
Effects of NH4+ priming on growth parameters.
Shoot fresh and dry weight
When compared to the control, the highest shoot fresh weight (112.7 g) was observed in T7, followed by T3, which was irrigated with normal water (41.9 g). The effect of the seed priming on the shoot fresh weight (SFW) (g/plant) of the tomato variety is shown in Table 4. However, when the NH4+ primed seeds were grown in saline stress, the shoot fresh weight increased significantly. In comparison to the control Nema (41.9 g), the maximum shoot fresh weight (g/plant) was recorded in treatment T8 (71.5 g). In the normal conditions, the primed tomato seeds responded favourably in the shoot dry weight (SDW). When compared to the control, T1 (50 mM NH4NO3, 12 h) had the lowest SDW value (5.8 g), followed by T2 (8.6 g). However, the highest shoot dry weight (g/plant), i.e. 14.7 g, was observed in treatment T3 (100 mM NH4NO3). The experiment findings revealed a variation among all the treatments carried out in saline conditions (Table 4). T5 (7.23 g) was found to exhibit the lowest dry biomass of shoot in freshwater versus the control (9.65 g).
Root fresh and dry weight
The analysis of variance revealed that the NH4+ priming caused a significant (p < 0.05) variation in root fresh weight (RFW) in both non-saline and saline environments. The highest root fresh weight (g/plant) was noticed in treatment T7 (22.7 g), compared to the control (9.4 g), while the lowest root fresh weight (5.5 g) was noticed in treatment T2. Moreover, when the seed priming and saline conditions were compared to the controls, treatment T3 exhibited the highest root fresh weight value (11.1 g) (g/plant), while treatment T4 was observed to have the lowest root fresh weight (5.67 g). In comparison to the control, T2 (0.58 g) had the lowest RDW in the normal conditions. While in the saline conditions, the NH4+ seed priming was observed to exert a significant effect on root dry weight. The maximum root dry weight (g/plant) was recorded in T7 (1.13 g) and the minimum root dry weight was observed in T5 (0.75 g) in cv, when grown in the control conditions (unprimed).
Leaf number and leaf area
The primed tomato seeds responded positively in terms of the number of leaves per plant in the normal conditions. Table 4 demonstrated that the leaf number was higher in T1 (50 mmol NH4NO3, 12 h) (92.4 g/plant), followed by T4 (91.8 g/plant), compared to the control. In contrast, in the saline environments, the NH4+ seed priming showed a progressive effect on the number of leaves, with the optimum leaf number per plant recorded in treatment T1 (82.2/plant), compared to the unprimed variant. Table 4 indicates the effect of seed priming and salinity on leaf area in the analyzed tomato variety. The highest leaf area was found in both T1 and T7 (18.5 cm2), followed by T3 (18 cm2), which was irrigated with normal water, compared to the control (14.3 cm2). However, when the primed tomato seedlings were grown in the saline conditions, the maximum leaf area (cm2) was recorded in T2 (18 cm2), compared to the control (unprimed).
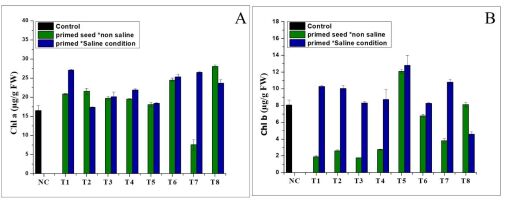
Effects of NH4+ priming on chlorophyll and carotene content
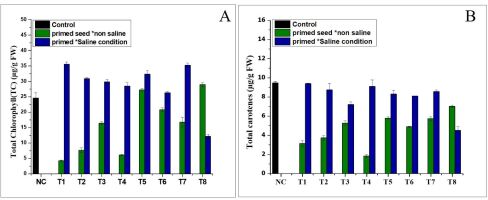
The results (Figure 1A) show that the chlorophyll a (Chl a) levels were higher in all the treatments than in the Nema control. In the normal conditions, the treatment produced the highest amounts of chlorophyll a (28.1 µg/g FW), followed by T4 (21.54 µg/g FW) in the saline conditions. Furthermore, the results revealed that the chlorophyll b levels increased only in a few treatments grown in the different conditions (normal and saline), compared to the control (Figure 1B). Treatment T5 was characterised by the higher chlorophyll b levels (12.06 and 12.8 µg/g FW) under seed priming in the normal and saline conditions, respectively. As shown in Figures 2A and 2B, NH4NO3 and (NH4)2SO4 significantly increased the total chlorophyll (TC) content in the different (normal and saline) conditions. Treatment T1 exhibited the highest amount of total chlorophyll (35.56 µg/g FW) in the saline conditions, followed by treatment T8 (28.9 g/g FW) in the normal conditions (Figure 2A). The findings shown in Figure 2B indicate that the total carotene was positively enhanced in the seed priming treatment in the normal and saline conditions, but the maximum total carotene was found in T4 (9.108 µg/g FW) in the seed priming treatment in the saline conditions (Figure 2B).
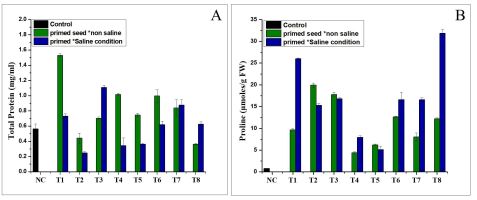
Effect of NH4+ priming on proline and total protein content
The result showed that the NH4+ priming without saline and under saline stress had a positive effect on the total protein content (Figure 3A). As shown in Figure 3A, the highest total protein concentration was found in treatment T1 (1.5299 mg/mL) in the normal water conditions, followed by T3 (100 mmol NH4NO3, 12 h). Under various abiotic stresses, including salinity, proline is the most frequently accumulated endogenous osmolyte. Proline improves crop tolerance to various abiotic stresses, particularly by protecting against harmful ROS effects. The content of free proline in the varieties varied significantly (p < 0.05). The proline content in the tomato leaves differed significantly between the NH4+ priming treatments. In the Nema variety, the treatments caused variable changes in the proline content (Figure 3B). Treatment T2 of cv. Nema (19.93 mol/g FW) in the saline conditions exhibited the highest proline content, compared to the control (Figure 3B).
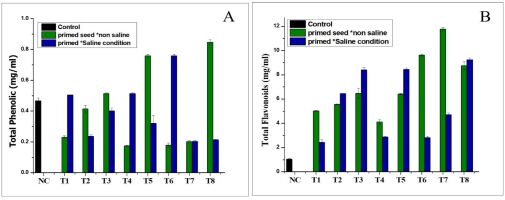
Effect of NH4+ priming on total flavonoids and total phenolic content
The total flavonoid content in the Nema variety was improved by the NH4+ priming treatment grown in the saline conditions, compared to the control (Figure 4A). The highest concentration was found in seed priming treatment T7 (11.7 mg/mL) in the normal conditions, followed by seed priming treatment T8 (9.2 mg/mL), which increased the total value of flavonoid content in the primed seeds in the saline conditions, compared to the control (1.03 mg/mL), as shown in Figure 4A. The statistical analysis of the data revealed that the seed priming treatment had essentially little effect on total phenolic contents in the non-saline (normal) and saline environments, as shown in Figure 4B. The results demonstrated that the total phenolic content was higher in treatments T8 (0.84 mg/mL) in the non-saline medium, while the total phenolic content in the seed priming treatment T5 was 0.75 mg/mL in the saline conditions, as shown in Figure 4B.
. Discussion
The current research demonstrated that two different ammonium sources (NH4NO3 and (NH4)2SO4) were employed as priming agents for seeds. This led to an enhancement in seed germination while simultaneously reducing the mean germination time (MGT) in tomato plants (Table 2). It is clear that the NH4+ priming with T5 increased the percentage of germination, whereas the NH4+ priming with T7 decreased the MGT in the tomato plant. In comparison to the unprimed seeds, the NH4NO3 and (NH4)2SO4 priming increased the germination percentage. According to a previous study (Pereira et al., 2022), nutripriming with 6 mmol NH4NO3 improved emergence synchronization (ES) caused by a decrease in the mean germination time in rice seedlings, and nutripriming with 2 mmol NH4NO3 improved total rice seedling growth. Vaktabhai and Kumar (2017) investigated tomato seedling vigour against salt stress. According to the findings, the halo-priming of tomato seeds with 1% or 2% KNO3 for 24 or 48 h could protect tomato seedlings from salt stress. Tomato seeds primed with 0.75% KNO3 were successful in enhancing seedling germination and vigour, as well as physiological and biochemical attributes in growth chamber and greenhouse conditions. As revealed in a study conducted by Hessini et al. (2013), NH4+ is more beneficial for the growth of S. alterniflora under high salinity than NO3. Changes in leaf area and leaf number were associated with changes in plant biomass. Hydro-priming and osmo-primin with Calcium Ammonium Nitrate (CAN) at 0.5% for 24 h stimulates maize crop development and vigour in aggressive environments, as reported by Khan et al. (2016). Based on the current findings, the leaf area and leaf number were improved in the different variants of NH4+ priming in the non-saline conditions. The positive responses to NH4NO3 and (NH4)2SO4 were also observed in the salinity conditions, but a better response was observed in 50 mmol NH4NO3, 24 h. Furthermore, in the saline conditions, the NH4+ priming induced a favourable response of the tomato plant biomass; when the NH4-N/total-N ratio in the nutrient solution was greater than 0.1, both vegetative development and fruit outputs in tomatoes were limited (Akl et al., 2003). On the other hand, an increase in total and fruit dry weight was shown when the ammonium percentage was 0.25 (Dong et al., 2004). According to Heeb et al. (2005), providing 0.1% of total-N in the form of NH4+ can improve fruit flavour by increasing glutamine and glutamate levels. Similarly, the current results showed that the fruit number and weight increased with the different NH4+ priming agent concentrations in the non-saline conditions, compared to the unprimed variant, whereas the NH4+ priming in the saline conditions gave the same positive response by increasing the fruit number and weight, but with a better response was shown in 50 mmol and (NH4)2SO4 for 12 h.
The present result (Figure 1, Figure 2) indicated that the photosynthetic pigments of the tomato plant were improved in the primed seeds in the normal conditions, compared to the unprimed seeds, and that chlorophyll b and total carotenes significantly increased under salt stress. Previous studies showed that NH4+ feeding on Spartina alterniflora in saline environments was associated with increased salt gland production, which led to a decrease in leaf mesophyll salt concentrations and enhanced photosynthetic activity (Hessini et al., 2011). Plant development and production are significantly impaired by salt stress. The salinity response to nitrogen nutrition types was studied in two major grass C4 species (sorghum and maize). Salt-stressed sorghum plants showed unchanged CO2 absorption when fed NH4+, a response strongly linked to photosystem II efficiency maintenance. The results clearly showed that grass species react differently to salt and different N types, demonstrating the vulnerability of salt-stressed maize plants to NH4+ feeding and an opposite behaviour of sorghum (de Souza et al., 2021).
Proline is synthesized in large quantities during salt stress and plays an important role in osmotic regulation, subcellular structure stabilization, and enzyme activities. It can also maintain cellular turgor pressure, which is responsible for cell growth (Sairam & Tyagi, 2004). Under growth chamber and greenhouse settings, tomato seeds primed with 0.75% KNO3 were successful in enhancing seedling establishment and vigour, as well as physiological and biochemical properties (Moaaz et al., 2020). The present study suggested that the proline content was raised in the NH4+ primed seeds grown in the non-saline and saline conditions (Figure 3). Spartina alterniflora, a halophyte, performed better when grown with NH4+ as an N source, and NH4+ advantages were correlated with improved antioxidant enzyme activity (Hessini et al., 2013).
The current research indicated that the primed seeds grown in the saline and non-saline stress had higher flavonoid content than the control seeds, and the phenolic content was higher in some treatments grown in the non-saline conditions. Nutripriming has been demonstrated to promote germination and seedling growth in some plant species; however, it is unclear whether this is simply due to the presence of nitrogen or if it might be attributed to the addition of supplementary nutrients such as magnesium and potassium.
. Conclusions
The current study demonstrated that using an ammonium priming agent favoured tomato seedling growth and development in both non-saline and saline environments. The 50 mmol (NH4)2SO4, 12 h priming resulted in the highest seed germination performance; however, all the treatments performed better than the unprimed variant. In addition, the growth parameter in tomato seeds was improved in both NH4NO3 and (NH4)2SO4 used at different concentration as NH4+ priming in the the non-saline and saline environment. This study justifies the use of NH4+ priming to solve the poor tomato seedling and emergence problem, but more research is needed to clarify the effect of NH4NO3 and (NH4)2SO4 as NH4+ priming in tomato plants because other factors and nutrition can play a role in seed germination and development.