. Introduction
Components of the endophytic phytobiomes establish mutualistic associations with their hosts where, in exchange of nutrients and habitat, they provide protection against various biotic and abiotic stresses. Their adaptation to life inside the tissues of other eukaryotes might trigger the capacity to synthesize novel bioactive secondary metabolites through the interaction with the host’s genome (Ekbic et al., 2022; Gakuubi et al., 2021; Nicoletti & Fiorentino, 2015; Rokas et al., 2020).
During the recent years, an explosion of research focused on the characterization of endophytes and their occurrence and functions, as well as on their aptitude as providers of ecosystem services have been observed (e.g. Dimitrova & Nacheva, 2021; Malinowski & Belesky, 2019; Sumi et al., 2022). Promises of a new green revolution aiming at the implementation of new techniques, sustainable solutions, and disruptive innovation trigger increasing interest of researchers all over the world in biodiversity and in bioprospecting endophytes as an alternative source of bioactive compounds to be used for industrial applications. Among others, members of Chaetomium and related genera represent a model of endophytic fungi as a rich source of unique bioactive metabolites that can be considered for diverse biotechnological deployment (Rao et al., 2023). This article particularly focuses on the species Arcopilus aureus (Chivers) X. Wei Wang & Samson (formerly Chaetomium aureum), following the finding of endophytic isolates from hazelnut (Corylus avellana L.) in the course of our ongoing studies carried out in Poland and in Italy.
. From Chaetomium to Arcopilus: A taxonomic excursus on the Chaetomiaceae and the identification of Arcopilus aureus
The family Chaetomiaceae (Sordariomycetes, Sordariales) was appointed to contain fungi that create non-stromatic sexual bodies with a membranaceous wall, covered by perithecial hairs similar to hyphal outgrows, fasciculate and evanescent asci, and single-celled, soft, brown or gray-brown ascospores; Chaetomium globosum Kunze was assumed as the type species (Kunze & Schmidt, 1817; Winter, 1885). Using classical methods, several attempts were made to study morphological details for species identification. The most relevant features taken into account were the characters of ascomata. Particularly, the structure of the ascomatal hairs was used as the most important feature in the first monographs, respectively describing 10 species (Zopf, 1881) and 28 species (Chivers, 1915). The appearance of ascomatal outgrows together with the shape and size of the sexual bodies and ascospores, in addition to the conspicuous characteristics, were used as the major separation characteristics by other taxonomists. According to these features, Skolko and Groves (1953) recognized 53 species, while Ames (1961) described 85 distinct species of Chaetomium.
Over the course of taxonomic redefinicion Chaetomiaceae, the most crucial amendment was made by Josef von Arx. Instead of focusing on the diverse perithecial hairs, he laid emphasis on the features of asci and ascospores, the presence of germ pores on ascospores, and the structureso f the ascomatal wall to distinguish species (Rong & van Warmelo, 1988). According to his taxonomical system, Chaetomium was kept for species creating ostiolate perithecia covered by relatively well-developed outgrows, and this feature allowed discriminating Chaetomium from other genera (von Arx et al., 1986).
Other taxonomical parameters, such as hyphal growth, colony color and size, and thickness of the hyphae within the colony, have been studied to distinguish species within Chaetomium (Plomley, 1959; Sharma & Pandey, 2010). Growth dynamics, colony form, morphology and appearance of mycelium and sexual spores varied greatly depending on the carbon source. Lignocellulose agar (LCA) and potato dextrose agar (PDA) have been pointed as the best media for culturing Chaetomium due to the flourishing growth of colonies and the appearance of all crucial taxonomical features (Sharma & Pandey, 2010). Chaetomium sensu stricto is characterized by the creation of globose, ellipsoidal to ovate or obovate perithecia, which most often are ostiolate, or non-ostiolate in some species. The teleomorph body wall is usually formed of textura intricata or epidermoidea stretch, or of textura angularis in some species; the perithecial outgrows are hypha-like, flexuous, undulate, coiled to simply branched or dichotomously branched, generally with verrucose stretch, and soft in some species. The asci are clavate or fusiform with eight biseriate or irregularly arranged sexual spores. The ascospores are limoniform to globose (erratical in a few species), bilaterally flattened, and usually more than 7 µm in length. An Acremonium-like asexual morph is formed in some species (Udagawa, 1960; Wang et al., 2016a, 2016b; Whiteside, 1957). Indeed, recent phylogenetic studies have shown that several fungi belong to the Chaetomiaceae which are only known for their asexual morphs and were previously classified in the genera Acremonium, Staphylotrichum, Humicola, or Trichocladium (von Arx et al., 1984; Wang et al., 2016a, 2016b).
Considering the microscopic features of the teleomorph for defining the species concept in Chaetomium has proven to be challenging, since they are widely evanescent and disappear before maturation of the sexual spores. Therefore, it is usually recommended to observe hyaline ascospores within asci. For spotting asci, diligent attention should be paid to the creation of ascomata. It is relevant to make slides from young perithecium at the precociousness of the culture, usually within two weeks, which we also did to identify our strains. When the specimens are getting mature for a longer time, most perithecia become matured and this makes it difficult to notice asci. For species that form non-ostiolate sexual bodies, hyaline young perithecia are mainly a good choice for ascus studies (Abdel-Azeem, 2020; Berkson, 1966; Wang et al., 2022).
The high phylogenetic heterogenicity of Chaetomiaceae determined the necessity to reclassify all taxa employing molecular tools. The ITS region of rDNA is recommended as the best primary barcode for identification and grouping of Chaetomium species (Lee & Hanlin, 1999). However, it could be assisted by a secondary barcode, and LSU (D1/D2 domains of the 28S nrDNA), rpb2 (partial RNA polymerase II second largest subunit), and tub2 (β-tubulin) sequences were proposed as additional barcodes. Nowadays, fifty genera incorporating 275 species are recognized in Chaetomiaceae; Chaetomium is the largest genus, accommodating 43 species (Wang et al., 2022).
Since Gustav Kunze officially introduced the genus Chaetomium (Kunze & Schmidt, 1817), remarkable progress has been made in the taxonomy of Chaetomiaceae. The comprehensive usage of molecular tools addressed the phylogenetic relationships for taxa with similar morphological features, with the imagery of five new genera, including Arcopilus; this name refers to the arcuate terminal outgrows of perithecia which characterize most of the members in this genus, including the type species A. aureus (Wang et al., 2016a).
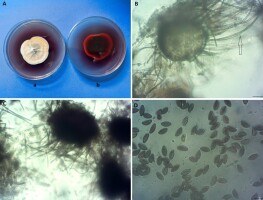
Despite the revolution following the employment of molecular tools, classical methods of identification should still be considered as the first step for classifying new strains. Our morphological observations were made after culturing the endophytic isolates of A. aureus from secondary branches of hazelnut Tv121 (from Motycz near Lublin, Poland) and A2269N (from the Astroni Nature Reserve near Napoli, Italy) on PDA (Difco) in darkness at 22 °C. After 7 days, the colonies were 30–36 mm in diameter, depicting yellow, orange to red color with red pigment diffusing into the medium (Figure 1A). The perithecia were 75–155 µm high and 80–150 µm in diameter, with an initially bright and thin wall (Figure 1B), then becoming brown and thick (Figure 1C). Characteristic arcuate hairs were observed, with an incurved apical part, circinate to coiled (Figure 1B). The ascospores were dark brown when mature, fusiform, elongate fusiform or limoniform, sometimes bilaterally flattened, with one or two apical germ pores, (8–10.5(–11) × (5–6(–6.5) µm) at both ends (Figure 1D).
Figure 1
Arcopilus aureus Tv 121. (A) colony on PDA (a-averse, b-reverse); (B) young perithecium with a thin wall and characteristic arcuate hairs (arrow); (C) old perithecium; (D) ascospores. Scale bars = 10 µm.
Identification of isolate Tv121 was also carried out through rDNA-ITS sequencing. To this end, total genomic DNA extraction from young mycelium taken from pure culture, PCR amplification, and sequencing were performed according to a previously published protocol (Zimowska et al., 2021). The original small subunit ribosomal RNA gene and rDNA-ITS (ITS1 – 5.8S – ITS2) sequence sourced in this study has been deposited in GenBank with the reference number OR178408. The identification of the fungal symbiont was acknowledged by checking the homology of this sequence with other sequences available in GenBank.
. Occurrence and ecological roles
With a worldwide distribution, species of the family Chaetomiaceae display elevated phenotypical and ecological diversity; they are mostly saprobic and occur in compost, soil, seed, dung, indoor environments, and rotting plant materials (Samson et al., 2019; Wang et al., 2016a). For its part, A. aureus has been diffusely mentioned from soil, dung, and other miscellaneous substrates (Abdullah & Azzo, 2015; Doveri, 2016; Kubatova, 2006; Kuter et al., 1983; Lee et al., 2019; Nakashima et al., 1991a, 1991b; Pornsuriya et al., 2010; Price et al., 1994; Quyet et al., 2018; Taniguchi et al., 1984; von Arx et al., 1986; Wang et al., 2013, 2016a). It has even been recovered from highly radioactive polluted soil and forest litter of the prohibited zone around the Chernobyl nuclear power plant (Ukraine) (Zhdanova et al., 2001) and from lead contaminated soil in Brazil (da Silva et al., 2018).
More significantly, A. aureus is characterized by a widespread occurrence as a plant associate. In fact, it has been reported from seeds of pepper and cucumber in the United States (Skolko & Groves, 1953), oats in Kansas (USA) and Canada (Conners, 1967; Hansing & Hartley, 1966), maize in the Chinese province of Liaoning (Gao et al., 2005), roots of sugarcane in Cuba (Hernández-Gutiérrez et al., 1995) and China (Raza et al., 2019), and bark of the cotton tree (Bombax ceiba) in Pakistan (Abbas et al., 2017). Some concern has been raised in China for its possible pathogenic aptitude, based on reports as a leaf spot agent on leaves of pineapple (Ananas comosus) in Hainan (Luo et al., 2012) and on the medicinal herb Pseudostellaria heterophylla in Guizhou (Yuan et al., 2021).
Moreover, this species has been identified as an endophyte in omnifarious geographic and ecological contexts. Particularly, there are records from stalks of bullgrass (Paspalum fasciculatum) in Costa Rica (Danielsen & Jensen, 1999), petioles of the Chinese fan palm (Livistona chinensis) in Thailand (Jiaojiao et al., 2016), black pine (Pinus nigra) in Spain (Martínez-Álvarez et al., 2016), various organs of grapevine in Brazil (de Faria Silva et al., 2022) and India (Dwibedi & Saxena, 2018), and from leaves and stems of the medicinal shrub Phlogacanthus thyrsiformis in India (Sharma et al., 2020). More findings have been reported from Yunnan (China), from leaves of Vaccinium dunalianum (Fan et al., 2020) and roots of the orchid Cypripedium flavum (Zang et al., 2004). In this region, an isolate from another orchid (Gastrodia elata) was identified as Arcopilus sp. (Duan et al., 2022); however, a blast in GenBank of its ITS sequence shows 100% homology with 18 isolates of A. aureus, besides an isolate of Arcopilus globulus. This latter match may have raised doubts in the authors about the correctness of the classification, which were dispelled considering that this species is now designated as a synonym of A. aureus (Wang et al., 2022). In a survey carried out on the longbranch frostweed (Crocanthemum canadense) in Nova Scotia (Canada), A. aureus was found to represent the most frequent endophytic associate colonizing every kind of tissues (root, leaf, lower and upper stem) in 56% of the examined plants collected at five out of six sites (Byers et al., 2021). Not too far geographically, this species was previously reported as one of four fungi colonizing roots of all the three plant species examined in the pine barrens of New Jersey, i.e. pitch pine (Pinus rigida), switchgrass (Panicum virgatum), and rosette grass (Dichanthelium acuminatum), implying a likely aptitude for a horizontal spread among plants thriving in the same ecosystem (Luo et al., 2017).
Arcopilus aureus is also part of the endophytic communities of trees of notable economic importance, such as olive (Olea europaea) (Nicoletti et al., 2020) and chestnut (Castanea sativa) (Nicoletti et al., 2021). An endophytic strain from the latter plant has been identified in Portugal as a prospective antagonist in the control of bark canker caused by Cryphonectria parasitica (Coelho et al., 2022). Moreover, an endophytic isolate from P. nigra reduced the disease progress resulting after inoculation of the canker agent Fusarium circinatum on Pinus radiata seedlings, indicating that it can find application in biological control of this pathogen (Martínez-Álvarez et al., 2016).
Another strain of A. aureus was previously identified as an effective biocontrol agent of the rice blast pathogen Magnaporthe grisea and the sheath blight pathogen Rhizoctonia solani both in vitro and in vivo. Particularly, its metabolites suppressed mycelial growth in R. solani and germination of spores and appressorium formation in M. grisea; moreover, they minimized the disease indexes of rice blast in the field and rice sheath blight both in the greenhouse and in the field (Wang et al., 2013). Culture filtrates of an isolate from Guzmania displayed strong inhibitory effects against Phytophthora infestans, Bipolaris sorokiniana, Fusarium culmorum, and F. oxysporum, while the bioactivity was moderate against Botrytis cinerea, B. fabae, Pyrenophora graminea, and Neocosmospora solani. These inhibitory effects persisted in the ethyl acetate extract of the culture filtrates (Linkies et al., 2021). Ethyl acetate, hexane, and methanolic extracts obtained from cultures of a soil strain determined some extent of inhibition in oospore formation in Pythium aphanidermatum (Pornsuriya et al., 2010). Finally, a Chinese strain of A. aureus was able to inhibit mycelial growth of Phytophthora capsici in dual cultures in vitro and to induce coiling of pathogen’s hyphae. Moreover, its fermentation broth and crude extract also almost completely inhibited mycelial growth of P. capsici, while treatment of pepper plants with fermentation broth activated defense enzymes, such as phenylalanine ammonia lyase, peroxidase, polyphenol oxidase, increasing their resistance. In addition, the fermentation broth significantly promoted growth of pepper seedlings, leading to an increase in fresh and dry weight (Liu et al., 2013).
. Secondary metabolites and other biotechnological aspects
By reason of the recent separation from Chaetomium, the production of secondary metabolites by A. aureus has been considered and reviewed together with other species of this genus (Zhang et al., 2012). Particularly, a huge research activity has been carried out on the biochemical properties of C. globosum; over 200 secondary metabolites have been isolated and identified from isolates of this species, including terpenoids, alkaloids, tetramic acids, diketopiperazines, steroids, xanthones, bis(3-indolyl)-benzoquinones, azaphilones, anthraquinones, pyranones, and orsellides, and many of them present interesting properties, such as anticancer, antimicrobial, antimalarial, cytotoxic, and antiviral activities (Dwibedi et al., 2023; Rao et al., 2023). In general, these fungi exhibit potent antimicrobial activities that encourage their use as biological control agents.
The data concerning secondary metabolites of A. aureus are more limited, and no information about this aspect was reported in the fundamental revision by Wang et al., (2016a). However, the analysis of the available literature indicates that the first bioactive secondary metabolite identified as a product of a strain of this species was oosporein (3,3′,6,6′-tetrahydroxy-5,5′-dimethyl-2,2′-bi-p-benzoquinone) (Lloyd et al., 1955). This compound, responsible for the red pigmentation of A. aureus cultures, was previously known as a product of other fungi, and it has been investigated as a phytotoxin and a mycotoxin affecting poultry (Cole et al., 1974; Manning & Wyatt, 1984); later on, it has been shown to exert antiviral and antifungal effects (Nagaoka et al., 2004; Terry et al., 1992), with the latter reported to increase at acidic pH (Taniguchi et al., 1984). Although it was not directly extracted, it was considered as likely responsible for the antifungal effect of culture filtrates and extracts from the strain examined in the previously mentioned work by Linkies et al. (2021). Moreover, it has been reported to exert protective effects in plants against aluminum stress based on its chelating properties (Haruma et al., 2022).
Another quinone derivative, cochlioquinol II, first reported as a phytotoxin from the Bermuda grass pathogen Bipolaris cynodontis (Lim, 1998), was found to be produced by an endophytic isolate from grapevine and reported to be responsible for the yellow pigmentation of this strain, along with riboflavin (de Faria Silva et al., 2022). Better known as pigments and for possible application as food colorants are compounds belonging to the polyketide class of azaphilones (Pimenta et al., 2021), which were discovered as products of an endophytic strain isolated in Morocco; particularly, isochromophilone IV and VII, sclerotioramin and sclerotiorin were extracted from cultures of this strain along with the tricyclic compound SB236050 and the new resorcinol derivative chaetorcinol (Kabbaj et al., 2015). Extracted from an endophytic strain from G. elata along with the biosynthetic analogs maristachone B, 8-zinniol methyl ether, and the novel arcopiniols A-C (Duan et al., 2022), zinniol is another product which was first characterized as a phytotoxin from several Alternaria and Phoma species (Cotty & Misaghi, 1984; Sugawara & Strobel, 1986); however, this biological effect has been questioned after more recent experimental assessments (Qui et al., 2010).
It is widely reported that many endophytic fungi can synthesize in vitro products originally characterized from their host plants (Nicoletti & Fiorentino, 2015). Among other endophytic fungi isolated from grapevine, a strain of A. aureus was characterized as the best resveratrol producer in an Indian study (Dwibedi & Saxena, 2018). This finding might have prompt biotechnological application, considering the notable beneficial effects of this polyphenolic flavonoid on human health, as well as its use as a pharmacophore.
Finally, an unidentified product (C31H35O8N), obtained from a strain classified as Chaetomium laterale var. diporum (currently a synonym of A. aureus), was discovered to almost totally inhibit mycelial growth of widespread pathogens, such as Sclerotinia sclerotiorum and Botrytis cinerea, at the concentration of 10 µg mL−1; however, its antifungal activity was much lower when assessed against other plant pathogenic fungi (Nakashima et al., 1991a, 1991b).
The limited extent of the available information concerning secondary metabolites of A. aureus calls for further insights, also in the aim of assessing possible chemotaxonomic relevance supporting species discrimination. Some of the above products were reported to exert phytotoxic effects; however, the spectrum of their bioactivity should be determined to establish if this property is general or specific, as well as if the doses released in vivo can effectively induce damage to the host plants. Moreover, clues concerning the fungitoxic effects also call for further assessments of the possible role of some products in defensive mutualism.
Besides the bioactive properties of secondary metabolites, biotechnological applications concerning isolates of A. aureus have not been substantially exploited so far. Some isolates have been reported as sources of xylanases (Iizuka & Kawaminami, 1969), cellulases (Ghora & Chaudhuri, 1975), and trehalase (Sumida et al., 1989), which is not so remarkable, considering that these are general properties of microbial strains recovered from any kind of environmental and geographical context. With reference to bioremediation, an isolate of A. aureus isolated in Brazil from semiarid soil with lead concentrations above regulatory limits exhibited better performance in laboratory assays in terms of fungal growth in a lead-containing substrate, capability to use pectin as a carbon origin, as well as basophilic and thermotolerant properties. Particularly, a reduction of free Pb in soil (61 and 54%, respectively) could be detected 60 days after inoculation. These findings support the use of this species as a potential tool for the bioremediation of contaminated sites (da Silva et al., 2018). Moreover, it has been proved to be highly tolerant to glyphosate (Hu et al., 2005).
. Conclusions
The examination of the available literature concerning A. aureus depicts its widespread geographic distribution and frequent occurrence as a plant symbiont, particularly in the form of an endophytic associate. In this respect, even the limited available data are indicative of a good adaptation to this ecological role, considering that it has been recovered from both roots and the above-ground parts of plants of various taxonomic affiliations. Some clues of the capacity to spread horizontally within heterogeneous ecological contexts portend an even wider and more pervasive occurrence, which stimulates further insights aiming at a more thorough assessment of its symbiotic interactions with the host.
The present paper represents the first report of the endophytic association of A. aureus with C. avellana, as inferable from the previous reports summarized in our recent review on endophytic fungi of this plant (Nicoletti & Zimowska, 2023). Indeed, the obtainable data on the occurrence and functions of endophytic fungi of hazelnut are scanty, and a good part of them refers to countries outside the European Union, such as China and Iran. Some published studies mainly concern the perspective of a pharmaceutical application, starting from the finding that a hazelnut endophytic strains of Penicillium aurantiogriseum is able to synthesize the antitumor drug taxol in axenic cultures (Yang et al., 2014). Ongoing studies concerning the metabolomic profile of our strains of A. aureus could bring new outcomes regarding the potential biotechnological exploitation of this fungus.
Most importantly, the association of A. aureus with hazelnut calls for further assessments in terms of its role in defensive mutualism. The antagonistic properties resulting from some published studies and our preliminary evidences support the possible implication of this fungus in protection of hazelnut from biotic adversities. Indeed, this crop is expected to face hard times in this respect after the announcement of phase-out of copper products in the European Union (Nicoletti et al., 2022). Hence, all components of the associated microbiome should be carefully evaluated for their contribution to plant health and fitness. In this respect, it is quite relevant considering that Chaetomium-like fungi have been already introduced in the management of plant diseases, and even some commercial formulates (e.g. Ketomium®;) have been released in several countries (Soytong et al., 2001).


