. Introduction
Ranunculus L. section Batrachium DC., hereafter referred to as Batrachium, is a monophyletic group of aquatic plants within the morphologically and ecologically diverse genus Ranunculus L., Ranunculaceae Juss. (Hörandl & Emadzade, 2012). In the latest worldwide taxonomic account of the section, 30 species were recognized (Wiegleb et al., 2017). The section is regarded as taxonomically challenging due to a limited number of taxonomically informative traits, considerable plasticity, hybridization, and polyploidization (summarized in Koutecký et al., 2022; Wiegleb et al., 2017). In earlier comprehensive taxonomic treatments of Batrachium (Cook, 1966; Pizarro, 1995; Tzvelev, 1998; Wang & Tamura, 2001; Whittemore, 1997), species delimitation was mainly based on extensive analysis of morphological characters, in some instances complemented by karyological, phytogeographic and ecological observations. However, due to differing views on the taxonomic importance of traits, species delimitations showed considerable divergence. A striking example is the differential use of the binominal ‘R. penicillatus’ in the monographic works of Cook (1966) and Pizarro (1995), respectively. Moreover, species boundaries are blurred by ongoing hybridization and introgression, which may not allow defining boundaries between newly created, mostly sterile hybrids and allopolyploid species originating from the same parentage.
The Mediterranean Basin hosts ca. 25,000 vascular plant species, of which about 5500 are endemic, rendering the region one of the major hot spots of global plant biodiversity (Lopez-Alvarado & Farris, 2022). The unique spatial patterns of the Mediterranean region in terms of a complex climatic and geological history, biotic interactions, migratory bird flyways, small-scale habitat heterogeneity, isolation effects on islands and mountain ranges, as well as thousands of years old anthropogenic pressure are considered driving forces of observed diversity (Nieto Feliner et al., 2023). Species of the section Batrachium mainly occurring in the northern hemisphere, show the highest diversity in Western Europe, followed by Eastern Europe, West Asia, and North Africa (Cook, 1966; Wiegleb et al., 2017). The Mediterranean region covers all these areas. Thus, we expected the region to be a rewarding research field for the study of taxonomic diversity and microevolution of the section. The taxonomic diversity of Mediterranean Batrachium is still poorly understood. Plants collected by early botanists in the region were regarded as conspecific with taxa described from temperate and boreal Europe, e.g., the heterophyllous taxa R. aquatilis L., R. peltatus Schrank, R. baudotii Godr., or R. penicillatus (Dumort.) Bab. More recently, Mediterranean plants were often treated as subspecies or varieties of northern species, e.g., R. aquatilis var. marizi (Coutinho, 1939) and R. peltatus var. microcarpus (Meikle, 1959). Simultaneously, deviant Mediterranean morphotypes were partly regarded as species in their own right, e.g., R. saniculifolius (Viviani, 1824), R. fucoides and R. leontinensis (Freyn, 1880), R. macranthus (Lojacono-Pojero, 1889), and R. castamonuensis (Dönmez, 2002).
West Mediterranean (Iberian) Batrachium was summarized by Cook (1986), Valdés et al. (1987), Velayos (1988), Pizarro (1995), and Cirujano Bracamonte et al. (2014). A closer look at their respective classification approaches shows profound differences in taxa delimitation. For this reason, the chromosome counts of Diosdado et al. (1993) cannot be properly assigned to taxa we consider valid nowadays. Taxa described by Maire (1964) from North Africa do not correspond to any of the aforementioned Iberian treatments. For the Central Mediterranean region, no synthetic approach except Pignatti (2017) is available. The treatments of Istrian (Englmaier, 2014), Corsican (Jeanmonod & Naciri, 2021), Sardinian (Desfayes, 2008), and Sicilian Batrachium (Giardina et al., 2007) all adopted divergent species concepts. East Mediterranean Batrachium was studied by Meikle (1959: West Asia; 1977: Cyprus), Cook (1965: Turkey), and Hand et al. (2011). In the 1990s, Batrachium diversity of the Aegean islands and continental Greece was comprehensively analyzed by Dahlgren (1991, 1992), Dahlgren and Svensson (1994), and Fiasson et al. (1997), including morphological, karyological, and phytochemical studies. Dahlgren’s findings were the basis for the Batrachium treatment in Flora Hellenica (Dahlgren, 2002). Dahlgren’s species concepts were strongly influenced by the circumstances typical for southern Sweden and Denmark, where she had worked before. In worldwide accounts (Cook, 1966; Wiegleb et al., 2017), Mediterranean Batrachium diversity was just touched upon in general terms. Several of the described and undescribed morphotypes known from herbaria remained unresolved. A striking example is R. fucoides Freyn, which was classified as ‘incertae sedis’ in both papers.
With the emergence of molecular techniques and a growing interest in integrative taxonomic approaches, the delimitation of Batrachium species started to become verified by Sanger sequencing of DNA markers (e.g., Bobrov et al., 2015, 2022; Lansdown, 2007; Telford et al., 2011; Zalewska-Gałosz et al., 2015), and genome size estimates (Koutecký et al., 2022; Prančl et al., 2018). Phylogenies based on the molecular characters showed a complex reticulate pattern of Batrachium evolution (e.g., Bobrov et al., 2015; Gebler et al., 2022; Koutecký et al., 2022), and allowed confirmation of hypotheses on phylogenetic relations beyond morphological analyses. Application of selected, multicopy DNA markers turned out to be useful in species and hybrid identification, however, for some species complexes, e.g., R. aquatilis/R. trichophyllus, R. peltatus/R. penicillatus agg. or R. baudotii, remained still indecisive due to low resolution. In these cases, the genome size estimates obtained by flow cytometry (FCM) are helpful since the genome size is characteristic of the individual taxa (Koutecký et al., 2022; Prančl et al., 2018). The FCM method, however, demands fresh tissues, and, for tracing hybridization and polyploidization events, it must be complemented by classical chromosome counts.
Contradicting nomenclatural approaches and a partly unresolved position of several Batrachium taxa indicate that classification solely based on morphology can often be misleading (Koutecký et al., 2022). Wiegleb et al. (2017) tried to develop an ‘enlightened’ morphological species concept for Batrachium. Species definitions were implicit and based on keys and species descriptions. Genetic and karyological information was used in addition if they supported morphological delimitation. The integration of molecular and morphological traits is nowadays more often used for species delimitation. In line with this practice, Hong (2020) proposed a new ‘gen-morph species concept’ that is theoretically objective and practically operable.
Considering the above, in this study, we apply an integrative morphological and genetic approach to selected European and Mediterranean Batrachium taxa. For the first time in Batrachium studies, we make use of novel high-throughput sequencing techniques that have opened new perspectives in plant taxonomic studies (Hörandl & Appelhans, 2015) and combine them with “classic” genetic markers generated by Sanger sequencing. We applied one of the reduced-representation sequencing techniques, namely the ddRADseq method, which generates genome-wide polymorphism data (Andrews et al., 2016).
The main aim of the present paper is to describe a new species, Ranunculus dahlgreniae, from Crete (Greece) in terms of integrative taxonomy. In addition, we aim to elucidate the morpho-genetic divergence within polymorphic species, such as R. baudotii, by comparing Batrachium phylogeny based on Sanger-sequenced nuclear (ITS) and cpDNA with the newly generated ddRADseq phylogenomic data. Finally, we shortly outline taxonomic and nomenclatural problems resulting from our work for the future treatment of Mediterranean Batrachium taxa.
. Material and methods
Plant material
Reference herbarium specimens of Batrachium taxa used for morphological and molecular studies are deposited in the Herbarium of the Institute of Botany, Jagiellonian University, Krakow (KRA). Plants collected in Denmark and Bavaria (Germany) in 2013 are deposited in the Oldenburger Landesmuseum für Natur und Mensch (LMO). Plant materials were collected in a responsible manner and exported in accordance with relevant permits and local laws.
Morphological evaluation
Morphological observations were made on fresh as well as herbarium material. Mediterranean specimens were collected during Mediterranean field trips to Croatia (2012, 2016, and 2018), Crete (2018), Spain (2021), and Portugal (2022). Measurements in the Description and in Table S2 refer to dried material. GW studied Batrachium specimens in B (Flora Hellenica collection, R. & E. Willing collection from Greece, Flora of Cyprus collection, personal collections R. Hand, N. Hadjikyriakou); GLM (incl. the Senckenberg Collection from FR); GOET (type material of R. fucoides), JE (material from Libya), LD (Gertrud Dahlgren collection, Flora Hellenica collection), LMO (own collection from Sardinia, J. Lambinon specimens from Corsica), MA (comprehensive Iberian collection), MAF (Iberian collection and private herbarium of J. Pizarro), STU (various Mediterranean specimens), and the private herbarium of B. Biel (Würzburg). JZG studied herbaria specimens in COI (Portuguese collection and Moritz Willkomm’s Historical Herbarium), K, H, and FRU. Additionally, Batrachium specimens were traced in virtual herbaria, photo portals, databases, and literature sources. In the present paper, only images retrieved from GBIF were used for taxonomic decisions. Observations and capture of images were performed using a dissecting binocular microscope (Zeiss Stemi SV 11, Germany) that was equipped with a digital camera (Canon EOS 760D).
Molecular survey
Genomic DNA extraction, PCR amplification, and Sanger sequencing
Fresh leaves of Batrachium individuals representing studied taxa were collected and stored in small plastic tubes filled with silica gel. Altogether, 191 Batrachium samples were analyzed at the molecular level; detailed information is summarized in Table S1.
Between 10 and 18 mg of dried plant material was used for DNA isolation. The plant tissue was ground to a fine powder using an MM 400 (Retsch) mixer mill and 3-mm tungsten beads. Total genomic DNA was extracted using the Plant Mini Kit (Qiagen), following the manufacturer’s protocol. The nuclear ribosomal Internal Transcribed Spacer region (including ITS1, 5.8S, and ITS2) was amplified and directly sequenced as described in Zalewska-Gałosz and Ronikier (2010, 2012). Additionally, two non-coding plastid spacers (cpDNA): rpl32-trnL and psbE-petL, were investigated to detect potential differences in the haplotypes among taxa. Amplification of cpDNA regions as well as sequencing was performed as described in Zalewska-Gałosz et al. (2009, 2010).
All sequences were submitted to GenBank (Table S1). Sequences were manually verified based on the forward reads or, in the case of intraindividual polymorphism, on the reads of both sequencing directions (forward and reverse). Alignments were prepared using BIOEDIT 7.0.5. (Hall, 1999). Polymorphic nucleotides in the ITS region were coded using IUPAC ambiguity codes. The two alignments, (1) ITS and (2) two concatenated regions of cpDNA, were trimmed according to the shortest sequence. Accessions that yielded identical ITS or cpDNA sequences were represented in the phylogenetic analyses by the single terminals, as described in Gebler et al. (2022), except for R. dahlgreniae. To obtain better clarity of phylogenetic visualization, intraspecies differences in sequences belonging to the same species, univocally, morphologically determined, and located in the same clade, were presented as a consensus sequence, using IUPAC code in polymorphic positions. Single ribotypes and haplotypes were identified by different numbers listed in Table S1. In cases where different ribotypes and haplotypes were observed within one species, it was specified with appropriate abbreviation (i.e., R. baudotii r1 cp1 and R. baudotii r3 cp3; Table S1). For R. dahlgreniae samples, the number of species-specific substitutions in the ITS region was calculated using the Fastachar program (Merckelbach & Borges, 2020).
Phylogenetic analyses based on the markers obtained in a Sanger sequencing
The ITS data were visualized as a phylogenetic network using SplitsTreeCE 6.0.0_alpha software (Huson & Bryant, 2006). The Hamming Distances Ambiguous States method (Hamming, 1950) was used to obtain a distance matrix. Splits were computed using the NeighborNet algorithm (Bryant & Moulton, 2004) with the Show Splits approach to obtain a Splits Network visualization.
For concatenated regions of cpDNA, the Bayesian analysis was performed in the MrBayes program vers. 3.2.7 (Ronquist et al., 2012). The evolutionary distances were computed using the HKY substitution model, which was chosen based on the lowest BIC (Bayesian Information Criterion) score computed in MEGA X (Kumar et al., 2018). The analysis was performed using two independent runs with four Markov chain Monte Carlo (MCMC) chains running for 105 generations. Trees were sampled after every 100th generation, and the first 25% of trees were discarded as the burn-in phase. The remaining trees were used for the construction of a 50% majority consensus tree. Ranunculus sceleratus L. was used as an outgroup, with three sequences acquired from GenBank: MW430773 (ITS), KC842129 (rpl32-trnL), and KC842059 (psbE-petL), trimmed to the required length prior analyses.
ddRADseq
RADseq libraries were prepared using the protocol (Suchan, 2020) and sequenced using Illumina HiSeq (150 bp single-end reads). Reads were demultiplexed with process_radtags from stacks 2.53 (Rochette et al., 2019). Further, analyses were performed with dDocent (Puritz et al., 2014) using mostly default parameters, except for the Minimum within individual coverage level to include a read for assembly (K1), which was set to 6 and the Minimum number of individuals a read must be present in to include for assembly (K2) set to 7. SNP calling was done with FreeBayes (Garrison & Marth, 2012) using the default parameters of the dDocent pipeline and assuming the diploidy of all samples. Genotypes with coverage below 5 or genotype quality below 20 phred were set to missing, and only the markers with less than 50% missing data were used in phylogenetic analysis.
The pairwise genetic distances between samples were calculated using function dist.gene() from ape (Paradis et al., 2022) using the pairwise deletion option, and the Neighbor-Joining tree was constructed using function nj() in ape. The tree was rooted with R. sceleratus. The robustness of the obtained relationships was tested with 1000 bootstrap replicates with boot.phylo() from ape. The tree was graphically visualized in the FigTree program v.1.4.4 (http://tree.bio.ed.ac.uk), and aesthetically improved in CorelDraw 2021.
. Results
Molecular survey
Phylogenetic relations among Batrachium taxa were reconstructed in three ways, based on different sets of molecular markers: (1) Bayesian analysis based on two, concatenated cpDNA spacers: rpl32-trnL and psbE-petL, (2) the ITS NeighborNet network, and (3) Neighbor Joining based on genetic distances between individuals obtained from SNP genotypes of ddRADseq data.
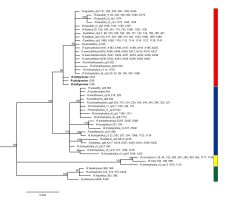
The alignment of two, concatenated chloroplast DNA spacers: rpl32-trnL and psbE-petL, was 1074 bp long. It contained 44 substitutions (28 of them potentially informative) and six 1–10 bp long indels. 23 haplotypes were distinguished among Batrachium samples based on the polymorphism detected (Table S1). The haplotype detected in R. dahlgreniae was very similar to haplotype cp3, which is widely distributed and shared by different Batrachium species, i.e.: R. aquatilis, R. fluitans, R. baudotii r3, R. peltatus, R. penicillatus A, R. saniculifolius, R. schmalhausenii, however, differed from cp3 by one mutational step in a position 865 of the alignment. In the phylogenetic tree based on plastid regions, four main, well-supported evolutionary groups were recognized (Figure 1).
Figure 1
Bayesian phylogenetic tree inferred from two cpDNA spacers: rpl32-trnL and psbE-petL and rooted with R. sceleratus (not shown).
Fifty-percent majority rule consensus tree is shown, numbers above branches are posterior probabilities, only values above 90 are shown. The scale bar represents substitutions/site. Haplotype and ribotype are indicated in taxa showing intraspecific variation. Colored boxes indicate clades and subclades. Ranunculus dahlgreniae is marked in bold.
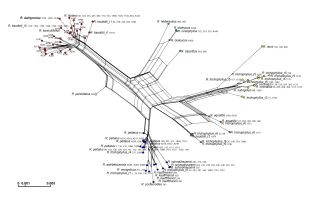
The ITS alignment of the whole data set was 573 bp long. These consisted of 43 substitutions (30 potentially informative) and two 1-bp long indels. Based on the polymorphism detected, 84 ITS ribotypes were distinguished (Table S1). The ITS sequence of R. dahlgreniae did not show any individual polymorphism. It was the most similar to the ribotypes of R. fluitans and R. baudotii r1 and r3 (but not r2). The R. dahlgreniae ribotype differed from the ribotype of R. fluitans by four substitutions (76th, 181st, 319th, 554th position of the alignment), from the ribotype of R. baudotii r1 by five substitutions (43rd, 48th, 181st, 192nd, 319th position of the alignment), and from the ribotype of R. baudotii r3 by three substitutions (86th, 181st, 319th position of the alignment). Adenine in positions 181 and 319 of the alignment was unique for R. dahlgreniae in comparison to ribotypes of the most similar species. Two species-specific substitutions were confirmed using the Fastachar program (Merckelbach & Borges, 2020). The phylogenetic ITS network showed five genetic groups comprised of more than one species. Additionally, two monospecific branches with the species of hybrid origin: R. aquatilis and R. penicillatus A were distinguished. These branches were located between groups to which their parents belonged (Figure 2). All species were spaced in a line with their taxonomic affiliations except for R. trichophyllus and R. baudotii. Six ribotypes of R. trichophyllus were distributed among three different groups. One of the groups comprised three R. trichophyllus ribotypes, although each of them had a different topology. Three ribotypes of R. baudotii were split between two groups (Figure 2).
Figure 2
NeigborNet of the ITS sequences of the Batrachium group. Ribotype or/ and haplotype are indicated in taxa showing intraspecific variation.
DNA numbers without species names represent R. saniculifolius. The colors of the dots correspond to genetic groups with congruent topology obtained in the other phylogenetic analyses presented in the study. Ranunculus dahlgreniae is marked in bold.
In ddRADseq, we obtained 8966 markers with less than half missing data, and the fraction of missing data per sample ranged from 0.06 to 0.64 (mean = 0.234, SD = 0.131).
Batrachium phylogeny based on three different molecular data sources
The topologies of Bayesian analysis (plastid DNA regions), NeighborNet Network (nrITS), and Neighbor-Joining (ddRADseq genotyping) were mostly congruent. Main genetic groups, marked in yellow, green, red, blue, and orange, were well-supported in all three analyses (Figures 1–3). Neighbor-Joining phylogenetic tree based on ddRADseq SNP data had the best resolution and turned out to be phylogenetically and taxonomically the most informative. In this tree, not only well-supported groups of taxa but also individual species were well-supported.
The first phylogenetic group (yellow) was formed by R. circinatus, R. rionii, R. subrigidus, and R. trichophyllus r5. Depending on the analysis, all samples of R. trichophyllus r5 were ascribed to this group (ITS Network, NJ ddRADseq) or split between ‘yellow’ and ‘blue’ groups, reflecting the inheritance of different haplotypes (cpDNA Bayesian tree). In NJ ddRADseq phylogeny, the yellow group was divided into five clades. Three of them corresponded with the accepted and widely recognized species, additional two reflected genetic differentiation within R. trichophyllus r5. Samples from Croatia, representing the Mediterranean R. trichophyllus (haplotype 10 and 11), are clearly separated from the individuals with haplotype 13, from Montenegro and Georgia (Figure 3; dark yellow squares; Table S1).
Figure 3
Neighbor-Joining (NJ) phylogenetic tree showing genetic relationships among Batrachium taxa, the approach based on 8966 markers generated using ddRADseq genotyping.
The tree was rooted with R. sceleratus (not shown). Grey dots indicating bootstrap support value ≥ 70%. Ribotype or/ and haplotype are indicated in taxa showing intraspecific variation. DNA sample ids are given next to the name of the taxon or ribotype/haplotype if indicated. Groups with congruent topology with the other phylogenetic analyses are marked in the same colors. Ranunculus dahlgreniae is marked in bold.
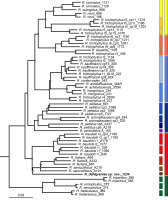
The second group (green) is formed by the West European species: R. ololeucos, R. hederaceus, R. omiophyllus, and R. tripartitus. This group is well-supported and was resolved in all three phylogenies.
The next group (red) was less congruent among the three phylogenies. The red group comprised core species: R. fluitans, R. baudotii, R. saniculifolius, and R. dahlgreniae sp. nov. In the cpDNA Bayesian tree, additional taxa were included here, i.e., R. aquatilis, R. schmalhausenii, R. peltatus, R. penicillatus A (sensu lato Koutecký et al., 2022), and the samples representing R. trichophyllus r3 and r6 with haplotype cp3 (Figure 1; Table S1). In the ITS Network, R. baudotii r2 was placed together with R. trichophyllus r6 in the other split than the ‘red’ group (Figure 2). In the NJ ddRADseq tree in the ‘red’ group, five clades were distinguished. Two of these reflected an intraspecific split in R. baudotii into two groups: the first comprised R. baudotii r1 occurring in Central and Northern Europe, and the second in which the Mediterranean R. baudotii r3 were grouped together with ribotype 2 from Central Europe. In this phylogeny within the ‘red’ group R. saniculifolius was resolved as a sister species to R. fluitans (Figure 3).
The ‘blue’ group was the biggest. In the ddRADSeq tree, which has the highest resolution, the group was split into two subclades, comprising mainly homophyllous R. trichophyllus-like plants (Figure 3; pale blue) and heterophyllous R. peltatus-like plants (Figure 3; dark blue). Both subclades were further subdivided into groups corresponding to individual taxa or intraspecific genotypes within R. trichophyllus, R. kauffmannii, and R. peltatus (discussed below). The ‘blue’ group in the ITS Network comprised the same taxa except for R. penicillatus A, which in the ITS network was separated into a monospecific group, placed between the ‘blue’ and ‘red’ groups reflecting its hydrogenous origin (Figure 2). The topology of the cpDNA Bayesian tree was in a great part congruent with the results described above, however, additionally, R. trichophyllus r5 samples from Croatia were included in the ‘blue’ group (Figure 2).
Ranunculus dahlgreniae against the background of Batrachium phylogeny
All three phylogenies identified R. dahlgreniae as a distinct lineage. Neighbor-Joining tree based on ddRADseq SNPs recovered R. dahlgreniae as a sister species to a lineage composed of R. saniculifolius and R. fluitans in a clade formed by R. baudotii, R. fluitans, R. saniculifolius and R. dahlgreniae (Figure 3). In the ITS network, R. dahlgreniae was placed in R. fluitans, R. baudotii r1, r3 and R. saniculifolius group (Figure 2). In Bayesian analysis based on cpDNA regions, R. dahlgreniae took a separate, basal position in the group formed by R. fluitans, R. peltatus, R. penicillatus A, R. schmalhausenii, R. aquatilis, R. trichophyllus r3, Central European R. trichophyllus r6, and R. baudotii, which except R. baudotii cp1 were separated in a subgroup (Figure 1).
Taxonomic treatment
Ranunculus dahlgreniae Zalewska-Gał., Wiegleb & Jopek, spec. nov.
Type: Greece, western Crete, Lefka Ori Mountains, a seasonal lake at Omalos Plateau, 1062 m a.s.l, 08.04.2018, J. Zalewska-Gałosz (holotype, KRA629945 (Figure S1); isotypes: KRA629943, KRA629946-52.
Paratypes: Greece, Crete: Ep. Kydonia, W. Kreta, Omalos hoogvlakte Levka Ori, in een poeltje in ca. 5 cm diep water wortelnd; 24.04.1967, Gradstein & Smittenberg 200A (L!). – Lefka Ori, ca. 40 m broad pool in SW. part of the Omala (sic!) plateau, 1200 m, Dahlgren B20 (Dahlgren, 1991; LD!!) – Kidhonia, Omalos plain, around the 2 small lakes near s border of the plain, partly dried out in summer, shallow water, wet and muddy lake side; 24.4./18.5.1994, Bergmeier & Matthäs 3739 (B!!). – Nomos Hamion, Ep. Kydonias, Omalos, Quellsee, 1055 m ü. NN, Flutrasen bzw. Trocken gefallene Quell-Löcher, 13.05.1997, Böhling 5684, 5685, 5686 (B!!).
Image:Figures S1–S3; achenes and receptacle details Figure 4.
Figure 4
Characters of achenes and receptacle of Ranunculus dahlgreniae based on herbarium specimens, gathered in the locus classicus at 8.04.2018. Scare bars 1 mm. (A) glabrous achenes with partly persistent styles; (B) slightly elongated, hairy receptacle with two immature achenes; (C) achene.

Diagnosis (see Table S2):
Ranunculus dahlgreniae is characterized by a combination of characters, which is not found in any other species within the section. These are the short pedicels, the small flowers (otherwise only found in dwarf forms of related species), and the relatively large achenes (in majority > 2 mm), with partly persistent styles (Figure 4). The species differs from all other recognized species in at least two independent characteristics: from R. saniculifolius it differs by the presence of filiform apical segments in intermediate leaves, the lack of triangular or elongated nectar pits, the larger achenes (Figure 4A,C), and the densely pubescent receptacle (Figure 4B); from R. baudotii it differs by the sparse branching, the shape of the intermediate leaves, lacking parallel-margined lobes, the short, mostly straight pedicels, the larger and largely unwinged achenes, and the subglobose receptacle, elongating only slightly in fruit; from R. peltatus it differs by the smaller size, the lower number of final capillary segments in submerged leaves, the lack of pyriform nectar pits, and the glabrous achenes, and from R. aquatilis it differs by the smaller size, the more deeply dissected laminar leaves, the shape of the intermediate leaves lacking basal filiform segments, the smaller flowers, and the glabrous achenes from the immature state onwards.
With R. saniculifolius, R. dahlgreniae shares the frequent annual life form, the triangular lobes in intermediate leaves, and the slightly elongating receptacles. With R. baudotii, it shares the whitish fleshy stem and the combination of glabrous achenes and a pubescent receptacle. With R. peltatus, it shares the intermediate leaves with apical filiform segments and the tendency to form crenulate or incised petals. With R. aquatilis, it shares the short non-elongating pedicels and the sometimes circular nectar pits. Overall, R. dahlgreniae is most similar to R. saniculifolius, followed by R. baudotii and R. peltatus. With respect to generally high-rated characteristics, such as the hairiness of the receptacle and the glabrous achenes, it resembles R. baudotii the most. The species does not share any significant trait with other Eurasian laminar-leaved species such as R. fucoides, R. tripartitus, R. ololeucos, R. schmalhausenii, and R. mongolicus (Nobis et al., 2017). Similarities with American laminar-leaved species such as R. lobbii and R. oblitus are of superficial nature.
Description:
Annual amphiphyte; aquatic state erect, spreading in the upper part, with laminar and capillary leaves (batrachid), terrestrial state prostrate with rigid, broadened capillary and laminar leaves. Shoots up to 60 cm long, diameter 1–3 mm, fleshy, whitish to pale green, glabrous. Laminar leaves present, alternate, 7–12(–15) mm long and 15–24(–35) mm wide, suborbicular to reniform, with 3(–5) primary lobes, dissected to 1/2 or 2/3 of the lamina length, secondary lobes 11–15(–17), hairy at the underside, margin crenate, rarely dentate, basal sinus of the lamina 120–180°; petiole 28–40(–60) mm long. Intermediate leaves sometimes present, divided into two or three petiolate leaflets with rigid filiform apical segments. Capillary leaves present, alternate, 20–60(–86) mm long, obconical to suborbicular, glabrous, green, segments flaccid or subrigid, divergent, middle part of the leaf well developed but shorter than lateral parts, reflexed; number of the lamina divisions 5–6, number of final segments up to 100; petiole 7–14(–50) mm long. Stipules for ca. 1/2 adnate to the petiole, glabrous, apex of free part obtuse to rounded, hairy at margins. Pedicels 30–58 mm long, mostly straight, sometimes recurved, glabrous. Sepals 5, up to 4 mm long, obovate to elliptical, with a blue edge, spreading, rarely reflexed. Petals 5, (3–)4–6.5 mm long, obovate, white with a yellow base, not contiguous during anthesis; sometimes of unequal size, or with one or two incisions up to 1/5 of petals length. Nectar pits 1 per petal, lunate or circular. Stamens 8–16. Carpels 15–30(–40), ripe achenes 1.8–2.2(–2.4) mm long, glabrous, unwinged except for the basal ventral part, beak lateral or subterminal, sometimes persistent. Receptacle subglobose to elliptical, hairy, hairs 0.75–1 mm long. – Chromosome no. 2n = 32 (Dahlgren, 1991).
Etymology: The species is named after Gertrud Dahlgren (1931–2009), who pioneered Batrachium research in the eastern Mediterranean region.
Habitat and ecology: The species inhabits freshwater bodies (astatic ponds) at an altitude between 1000 and 1200 m asl. The Omalos plateau is made of calcareous rock.
Status and phytogeography: The species is indigenous to the island of Crete. Whether or not the species is endemic is to be decided. Similar morphotypes sharing the glabrous achenes and the pubescent receptacle have been observed elsewhere in Greece. For these plants, neither complete descriptions nor genetic data are available so far. Locations are concentrated in regions adjacent to western Crete, such as Peloponnese and the Cyclades Islands.
Greece: Thessalia, Larisa, R. & E. Willing 177.540–177.570 (B!!), Herb. Willing 3770 (B!!), achenes not completely glabrous; Peloponnes, Jagel (1992; Jagel & Nikolopoulou, 2023), as ‘R. peltatus subsp. fucoides’, with larger petals and longer pedicels; Peloponnes, Elafonisos, Aleksejew s.n. (STU!!); Peloponnes (Blaich, 2018), as ‘R. trichophyllus’, with larger petals; Aegean Islands; Delos and Mykonos, Dahlgren (1991, specimens B4 and B16, LD!!), as R. peltatus subsp. baudotii and R. peltatus s.l., respectively; Naxos, Böhling 4034 (STU!!); Paros, Raus & Schiers, s.n. (B!!), Milos, Biel MI 20.14, 20.124 (private collection B. Biel!), as ‘R. peltatus subsp. peltatus’.
Key to laminar leaved species of the East Mediterranean region
1 Laminar leaves deeply dissected (to 2/3 or 3/4 of the lamina); stem and leaves fleshy, often whitish; achenes glabrous ........................................................ 2
[1*] Laminar leaves mostly only dissected to 1/2 of the lamina; stems and leaves not fleshy; achenes hairy, at least in juvenile state ........................................................ 3
[2] Achenes dorsally and ventrally conspicuously winged, style caducous; intermediate leaves tripartite, symmetric, lobes narrow with parallel margin, rounded at apex; pedicels longer than 50 mm, elongating in fruit, mostly recurved; petals 5.5–13 mm long; receptacle considerably elongating in fruit; in brackish coastal waters, mostly along coasts, also in sulphate-rich waters inland, e.g., Corfu, northern Greek coast, northern Aegean Islands ........................................................ R. baudotii Godr.
[2*] Achenes unwinged, except for the lower ventral part, a style sometimes persistent; intermediate leaves with 2–3 cuneate lobes, sometimes with rigid filiform apical segments; pedicels shorter than 50 mm, slightly recurved; petals (4–)5–6.5 mm long, receptacle slightly elongating in fruit; freshwater pools, Crete, to be confirmed for Greek mainland, Peloponnese, Greek Aegean Islands ........................................................ R. dahlgreniae Zalewska-Gał., Wiegleb & Jopek
[3*] Receptacle puberulent, hairs 0.2 mm long; laminar leaves often truncate at base, basal sinus 120–180°, intermediate leaves with cuneate lobes; capillary leaves with up to 100 final segments; pedicels elongating in fruit, recurved; nectar pits lunate, horseshoe-like or triangular; in pools and ditches, in fresh and brackish water; also in small streams ........................................................ R. saniculifolius Viv.
[3] Receptacle densely pubescent, hairs 0.5–1 mm long; laminar leaves rarely truncate at base; basal sinus 0–150°; intermediate leaves without cuneate lobes; capillary leaves with up to 250(−400) final segments; pedicels not elongating or elongating before fruit; nectar pits circular or pyriform ........................................................ 4
[4] Intermediate leaves with apical filiform segments; secondary lobes of laminar leaves rounded; capillary leaves with up to 200(−400) final segments; pedicels elongating before flowering, straight or slightly recurved; nectar pits pyriform (except in juvenile and annual forms); in various water types, European-West Siberian species, in the region on Greek and Turkish mainland, southern distribution insufficiently known ........................................................ R. peltatus Schrank
[4*] Intermediate leaves with basal filiform segment, asymmetric; secondary lobes of laminar leaves acute; capillary leaves with up to 100 final segments; pedicels not elongating, mostly straight; nectar pits circular or cup-shaped, in freshwater ponds and ditches, temperate European species, in the region only in northern Greece ........................................................ R. aquatilis L.
. Discussion
The new species R. dahlgreniae has never been formally described before; thus, synonyms are not available. Its specific morphological features were either not recognized or not regarded as important. Even though R. dahlgreniae is most similar to R. saniculifolius, it was never included in descriptions of that taxon in comprehensive taxonomic treatments. Cook (1966) described R. saniculifolius based on a specimen from Cyprus as a species with few (2–5) large achenes (2.5 mm long) and a glabrous receptacle. Achene number and size were later corrected (Cook, 1967), but the description does not fit either. Pizarro (1995) reported 15–65 achenes of intermediate size (1.5–2.0 mm). His plants had a glabrous or sparsely short-haired receptacle, the image presented has no similarity with R. dahlgreniae. Wiegleb et al. (2017) described R. saniculifolius as having hairy or glabrous achenes of a length of 1.2–2.5 mm, and a glabrous or slightly hairy receptacle. This included all Mediterranean morphotypes of that species, but also other taxa such as R. fucoides Freyn and R. peltatus var. microcarpus Meikle. Still, it did not cover typical features of R. dahlgreniae in full, such as the short pedicels and the densely pubescent receptacle. Likewise, the new species is not covered by the descriptions of R. baudotii and R. peltatus, respectively, as outlined by Cook (1966), Pizarro (1995), and Wiegleb et al. (2017).
Nevertheless, R. dahlgreniae has been mentioned in the regional literature under some misapplied names. It was identified as ‘R. peltatus s. l.’ by Dahlgren (1991, p. 200). It is included in ‘R. peltatus subsp. saniculifolius (Viv.) Dahlgren (2002, p. 66), which can be inferred from the shape of intermediate leaves (‘capillary segments’), achene size (up to 2.4 mm), and the formation of a basal ventral wing. It may have been identified as ‘R. aquatilis L.’ by Bergmeier & Abrahamczyk (2008, p. 445, occurring together with ‘R. peltatus subsp. saniculifolius’) from Lasithi/Mirambelou, at 280 m asl. The chromosome count (2n = 16) reported by Montmollin (1984) as ‘R. peltatus subsp., from Omalos, 1100 m asl’ may refer to a homophyllous species found in that region (see the specimen Gradstein & Smittenberg 200A, found in GBIF, from L).
The species R. fucoides Freyn, in Lange & Willk., Prodr. Fl. Hispan. 3(4): 912 (1880) was included as a subsp. of R. peltatus Schrank by Muňoz-Garmendia (1985) and synonymized with R. peltatus subsp. saniculifolius, as denominated by Cook (1984). This had enormous negative consequences on Batrachium taxonomy, as the name ‘fucoides’ now spread to areas where the taxon does not occur. We studied specimens of R. fucoides in MA and GOET. It is a distinctive species, characterized by the large flowers, the short rigid fleshy capillary leaves, and the long, mostly coiled pedicels. It was correctly separated from R. peltatus, R. baudotii, and R. saniculifolius at the subspecies level by Valdés et al. (1987, Fig. on p. 112), Diosdado et al. (1993, Fig. 274, p. 238) and Cirujano Bracamonte et al. (2014) and should be re-established as a separate species.
We could not fully solve the problem of the identity of R. saniculifolius. Even after the separation of R. fucoides and R. dahlgreniae as separate species, the taxon remains heterogeneous. Our samples, which were collected in the West Mediterranean region, are genetically related to R. fluitans and R. baudotii and showed big genetic heterogeneity (Figure 2). Even though the morphology of central and east Mediterranean plants is similar, additional studies will be necessary for the future to confirm their affiliation to the R. baudotii-R. fluitans clade.
We assume that the following unresolved taxa described from the region are extreme morphotypes of Ranunculus peltatus (see remarks of Parolly et al., 2007) and do not belong to the new species: R. kastamonuensis Dönmez (from Turkey) is a slender plant with a few large achenes; it is also found in northern Greece. R. peltatus var. microcarpus Meikle (from Cyprus, Meikle, 1977) is a slender plant with many small achenes; it is also found on Rhodes, Sicily, and in southern Spain, Portugal, and Morocco (see Pizarro, 1988). R. tripartitus G. Dahlgren non DC. (from Greece, Mykonos, also found in Milos and in central Greece) is a small hexaploid plant similar to R. peltatus, both with respect to morphology and karyotype (Dahlgren, 1991; Dahlgren & Svensson, 1994).
The phylogenetic relations within Batrachium are consistent with those obtained in the previous molecular studies (Bobrov et al., 2015; Koutecký et al., 2022; Prančl et al., 2018). However, the application of a new, high-throughput genome-wide analysis ddRADseq and sampling of taxa not included in the previous molecular studies allowed expanding the knowledge about phylogenetic relationships within the section and describing Ranunculus dahlgreniae, a species new to science.
Our study supports the hypothesis of Wiegleb et al. (2017) that Ranunculus ololeucos belongs to a West European clade comprising R. omiophyllus, R. hederaceus, and R. tripartitus (Figure 1, Figure 2). The clade is made up of small amphibious species, which are partly lacking capillary leaves (R. hederaceus, R. omiophyllus). All species except R. hederaceus have large, free, membranous stipules. On the upper side of the laminar leaves are frequently black spotted along the veins. Velayos (1988) had regarded R. ololeucos as conspecific with R. saniculifolius, however, in light of our results, this hypothesis must be rejected. Most probably, the plants he studied were of hydrogenous origin.
Progress was made in understanding the taxonomy of some complex species. It was recognized that R. peltatus does not only have a northern sibling species (R. schmalhausenii), but also a southwestern one (the Iberian R. peltatus). As can be inferred from Figure 1, the Iberian ‘R. peltatus cp6’ is even more distinct from temperate R. peltatus than the northern species R. schmalhausenii. In this case, genetic differences are only partly reflected by morphological divergence. Iberian R. peltatus is, on average, more slender than temperate R. peltatus, the laminar leaves are more often 5-lobed with entire margins, in addition, more often black dots are found on the upper side along the veins, and the submerged leaves are more often short and congested. In contrast to the situation in Scandinavia, where we find a 100 to 200 km wide transition zone north of 60° northern latitude between southern R. peltatus and northern R. schmalhausenii, no such transition zone exists on the Iberian peninsula. Most plants south of the Pyrenees and the Cantabrian Mountains are Iberian R. peltatus. However, plants resembling the Iberian form have also been collected in southern France along the Rhone Valley.
It has been known for a long time that R. baudotii is a complex species, as reflected by the common synonym R. confusus Godr. and R. marinus (Fr.) Fr. Wiegleb et al. (2017) assumed that there is a northern morphotype and a southern morphotype, with a slightly different stipule and achene shape. Moreover, the existence of inland forms with partly intermediate characters was mentioned, which was later confirmed by Koutecký et al. (2022). Genetic analysis showed that the current picture is more differentiated. The northern forms may go further southwards, both along bird flyways in inland areas and along the coasts. Morphologically inland forms rather resemble the southern form more than the northern one. This observation is well-supported by phylogeny based on ddRADseq data, where samples of R. baudotii from Croatia and from southern France were clustered with central European inland ribotype r3 (Figure 3). However, the southern form may not be homogenous but may be divided into a western form with larger flowers and an eastern form with smaller flowers. The exact distribution of the forms and their relationships must be studied in more detail in the future.
The molecular findings fully support our previous assumption that Mediterranean Batrachium may comprise more species than is currently known. From the combined studies, it became evident that the well-known Batrachium flora of Central Europe (Germany, Poland, Czech Republic) does not have many species in common with the East Mediterranean region. Only R. rionii, R. trichophyllus, and R. baudotii are regularly found in both regions. The latter two are represented by markedly different ribo- and haplotypes in the respective regions. This observation may hold for the Western Mediterranean region. Other ‘good species’, associated with the Mediterranean and mentioned by Wiegleb et al. (2017) as uncertain are R. fucoides Freyn (see above), R. lutarius (Rev.) Bouvet, and R. pachycaulon (Nevski) Luferov. The status of R. sphaerospermus Boiss. et C.I. Blanche as a separate species is undisputed nowadays. Phylogenetically this species originates from the ‘yellow’ group, which can be hypothesized based on the ITS sequence (Hörandl & Emadzade, 2012). R. sphaerosphermus is closely related to Mediterranean R. trichophyllus r5. In the RADSeq tree, R. trichophyllus r5 was split into two clades (Figure 3). Distinct evolutionary lineages within R. trichoplhyllus that are otherwise morphologically indistinguishable have been recognized before (Bobrov et al., 2022; Koutecký et al., 2022; Prančl et al., 2018; Zalewska-Gałosz et al., 2015). Our research shows that in the Mediterranean area, there are further, phylogenetically distinct genotypes in this wide-range morphospecies (e.g. r5, Mediterranean r6). The factors shaping this variability, as well as its taxonomic implications, require further research.
New species have also been discovered elsewhere. Recently, R. oblitus was described from South America (Wiegleb et al., 2022), which is related to North American R. trichophyllus. In that paper, another new species related to R. subrigidus W.B. Drew was suggested by genetic data but could not yet be morphologically verified due to a lack of well-preserved material. Furthermore, the so-called R. trichophyllus from New Zealand (Wiegleb et al., 2017) could be identified as an undescribed taxon close to R. baudotii (A.A. Bobrov, G. Wiegleb, P. Garnock-Jones, unpublished data). Overall, none of these newly described taxa can be regarded as ‘cryptic species’ in the sense of Prančl et al. (2018). They may be called ‘semi-cryptic’ species. As soon as sufficient material is collected and analyzed, the case will become evident. We estimate that the total number of recognizable species in Batrachium is closer to 40 than 30, as assumed by Wiegleb et al. (2017).
Comparison of the Batrachium phylogeny resolved with different methods highlights the utility of reduced-representation sequencing for resolving phylogenetic relationships in non-model organisms with reticulation and recent divergence.
. Supplementary material
The following supplementary material is available for this article:
Table S1. Samples of Ranunculus section Batrachium included in the DNA studies.
Figure S1. Holotype Ranunculus dahlgreniae preserved in KRA.
Figure S2. (A) Crete, Lefka Ori Mountains, a seasonal lake at Omalos Plateau, locus classicus of Ranunculus dahlgreniae sp. nov. (B) Flower and a floating leaf of Ranunculus dahlgreniae in the field. Photographs taken in April 2016 by Jenny Neal, reproduced with permission of the author.
Figure S3. Ranunculus dahlgreniae in shalow parts of the lake. Photographs taken in April 2016 by Jenny Neal, reproduced with permission of the author.
Data availability
The Sanger sequences reported in this paper have been deposited in GenBank databases (www.ncbi.nlm.nih.gov/genbank/). The datasets used and/or analyzed during the current study are available from the corresponding authors upon request. Herbarium specimens of R. dahlgreniae are preserved in KRA.


