. Introduction
The family Aspleniaceae (class Polypodiopsida) is one of the most species-rich groups of ferns (Lin & Viane, 2013). Depending on classification, it includes from ca. 730 species (Hassler, 2023; Lin & Viane, 2013; Mabberley, 2008; Pteridophyte Phylogeny Group I [PPG I], 2016) to more than 3,100 (POWO, 2023).
The Aspleniaceae are a cosmopolitan group of spore-bearing vascular plants on all continents except Antarctica (Hassler, 2023; POWO, 2023). Many of them are terrestrial (growing in the ground) but some are epiphytic. They are found on the floor of tropical forests, on river banks, walls of ravines, caves, old buildings, and in rock crevices, as components of alpine vegetation (Kaplan et al., 2016; Kramer & Viane, 1990; Ohlsen et al., 2015; Smith et al., 2006).
The distribution of species of this family is uneven: ca. 30% in the Neotropics, 22% in Africa, 33% in Asia, 10% in the Pacific, including Australia, and only ca. 5% in Europe (Kramer & Viane, 1990). The European flora contains 31 species of the family Aspleniaceae (Valentine & Moore, 2007), while in East-Central Europe, it is represented by ca. 25 taxa, including about a dozen hybrids (e.g., Jäger & Werner, 2005; Mirek et al., 2020; Reichstein, 1984; Szczęśniak et al., 2017). Many species are also subdivided into taxa of lower ranks (e.g., Danihelka et al., 2012; Didukh et al., 2000; Ekrt & Štech, 2008; Hassler, 2023; https://floraweb.de/; Jäger & Werner, 2005; Mosyakin & Fedoronchuk, 1999).
The systematics of the family Aspleniaceae is very complicated and still raises doubts (Xu et al., 2020, 2021). In the late 20th century, 2 approaches to classification of this group were developed: (1) the family includes only one genus Asplenium, consisting of all the closely related species (e.g., Kramer & Viane, 1990; POWO, 2023), and (2) Asplenium is distinguished from several so-called satellite genera (e.g., Schneider et al., 2004). Currently, on the basis of molecular techniques, the prevailing opinion is that the Aspleniaceae consist of two genera: Asplenium (including e.g., the former genera Antigramma Presl, Camptosorus Link, Ceterach Willd., Ceterachopsis (J. Sm.) Ching, Diellia Brack., Diplora Baker, Holodictyum Maxon, Loxoscaphe T. Moore, Phyllitis Hill, Pleurosorus Fée, Schaffneria Fée ex T. Moore, Scolopendrium Adans., and Sinephropteris Mickel.) and Hymenasplenium (Christenhusz et al., 2011; Hassler, 2023; PPG I, 2016; Xu et al., 2020). According to POWO (2023), the Aspleniaceae comprise also members of the former families Athyriaceae, Blechnaceae, Desmophlebiaceae, Hemidictyaceae, Onocleaceae, Rhachidosoridaceae, Thelypteridaceae, and Woodsiaceae, but that approach is not broadly accepted. All the European species of the family Aspleniaceae are currently included in the genus Asplenium (Christenhusz & von Raab-Straube, 2013; Hassler, 2023).
One of the important research directions providing valuable information used to solve taxonomic problems is concerned with the description of spore morphology. This direction was initiated in the 1960s (Nayar & Devi, 1964) but it developed intensively as a result of the application and progress of scanning electron microscopy (Puttock & Quinn, 1980; Viane & van Cotthem, 1977; Wei & Dong, 2012). Studies of spore morphology supply valuable information not only for taxonomic research but also for paleobotany, phylogenesis, and biogeography (e.g., Gonçalves de Freitas et al., 2015; Homes et al., 2015; Mazooji & Salimpour, 2014; Sun et al., 2010). The high value of spore morphology in taxonomic and phylogenetic research is also emphasized by authors who study other groups of ferns, e.g., the families Davalliaceae, Dennstaedtiaceae or Ophioglossaceae (Olejnik et al., 2018; Wang et al., 2015; Yañez et al., 2017).
Spore morphology in the Aspleniaceae was also investigated in many studies, which provide valuable data (e.g. Braggins & Large, 1990; Curto et al., 2012; Dong et al., 2012; Gabancho et al., 2006; Ganem et al., 2013; Irfan et al., 2022; Lashin, 2012; Lorscheitter et al., 2002; Morbelli & Giudice, 2005; Regalado & Sanchez, 2002; Shah et al., 2020; Tryon & Lugardon, 1991; Wei & Dong, 2012). Most of the publications concern one or several species and provide morphological descriptions of spores. Few studies concern more than 10 species or cover larger geographic regions (Dai et al., 2005; Lorscheitter et al., 2002; Puttock & Quinn, 1980; Wei & Dong, 2012), aiming to describe spore morphology and sometimes attempt to distinguish morphological types (Ferrarini et al., 1986; Pangua & Prada, 1988).
This study aims to (1) describe in detail spore morphology in Central and Eastern European species of the Aspleniaceae; (2) attempt to use spore characteristics in taxonomic analyses; (3) create a key to species identification on the basis of ornamentation of spores of the family Aspleniaceae from Central and Eastern Europe.
. Material and methods
Plant material
The spores used in this study originate from dried specimens from the Herbarium of the Department of Systematic and Environmental Botany (formerly Department of Plant Taxonomy) of Adam Mickiewicz University, Poznań, Poland (POZ), Herbarium of the M. G. Kholodny Institute of Botany of the National Academy of Sciences of Ukraine in Kyiv (KW), Herbarium of the Uzhhorod National University (UU) and from material collected specifically for this study (Table 1, see also: https://amunatcoll.pl/). In total, we examined spore samples of 10 species of the genus Asplenium from 57 locations (see Table 1). This study did not take into account about a dozen taxa that are interspecific hybrids (e.g. A. × alternifolium Wulfen or A. × centovallense D. E. Mey) and species with poorly-studied distribution ranges or an ambiguous taxonomic status (e.g., Ceterach javorkeanum (Vida) Soó). The hybrid taxa are usually found in only single localities and produce no spores or only abortive spores, so the plants are infertile (por. Hornych & Ekrt, 2017).
Table 1
Origin of spore samples of ferns of the family Aspleniaceae.
| Asplenium species | Location, habitat | Collection date (DD.MM.YYYY) | Collector* |
|---|---|---|---|
| A. adiantum-nigrum | Chorna Hora, near Vinohradiv, Transcarpathian region, Ukraine; stony places | 07.06.2005 | M. Shevera (KW s.n.) |
| A. adiantum-nigrum | Gozdnik II, Sudetes Foothills, Lower Silesia region, Poland; crevices of serpentine rocks | 11.10.2013 | E. Szczęśniak |
| A. adiantum-nigrum | Gozdnik I, sunny site, Sudetes Foothills, Lower Silesia region, Poland; crevices of serpentine rocks | 11.10.2013 | E. Szczęśniak |
| A. adiantum-nigrum | Gozdnik I, shaded site, Sudetes Foothills, Lower Silesia region, Poland; crevices of serpentine rocks | 11.10.2013 | E. Szczęśniak |
| A. adiantum-nigrum | Kamienny Grzbiet, shaded site, Lower Silesia region, Poland; crevices of serpentine rocks | 11.10.2013 | E. Szczęśniak |
| A. adiantum-nigrum | Kamienny Grzbiet, Lower Silesia region, Poland; crevices of serpentine rocks | 11.10.2013 | E. Szczęśniak |
| A. adulterinum | Kiełczyn Hills, Sudetes Foothills, Lower Silesia region, Poland; crevices of serpentine rocks | 11.10.2013 | E. Szczęśniak |
| A. ceterach | Botanical Garden in Wrocław, Lower Silesia region, Poland; self-sown within Botanical Garden | 10.09.2021 | E. Szczęśniak |
| A. ceterach | Simeiz, Crimea region, Ukraine; stony places | 12.09.2015 | L. Ryff |
| A. ceterach | Gurzuf forestry, in Sanipernus juniper forest, Crimea region, Ukraine; stony places | 07.1973 | Yu. R Shelyah-Sosonko, G. Kuhkovitsa, Ya Didukh (KW s.n.) |
| A. cuneifolium | Gozdnik Przemiłów, Sudetes Foothills, Lower Silesia region, Poland; crevices of serpentine rocks | 11.10.2013 | E. Szczęśniak |
| A. cuneifolium | Przemiłów, sunny site, Sudetes Foothills, Lower Silesia region, Poland; crevices of serpentine rocks | 11.10.2013 | E. Szczęśniak |
| A. cuneifolium | Świątniki, Wrocław County, Lower Silesia region, Poland; crevices of serpentine rocks | 11.10.2013 | E. Szczęśniak |
| A. cuneifolium | Świątniki, shaded site, Wrocław County, Lower Silesia region, Poland; crevices of serpentine rocks | 11.10.2013 | E. Szczęśniak |
| A. cuneifolium | Summit of Radunia Mt (Sępia Góra) near Sobótka, Wrocław County, Lower Silesia region, Poland; crevices of serpentine rocks | 20.09.1956 | H. Piotrowska (POZ-V-0001760) |
| A. cuneifolium | Valley of river Jihlava near Mohelno, western Moravia, Czechia; crevices of serpentine rocks | 1924 | R. Dvořak (POZ-V-0001764) |
| A. lepidum subsp. haussknechtii | Kos’mo-Damianovskiy Muzhskoy Monastyr (Monastery), Crimea region, Ukraine; stony places | 07.10.2012 | L. Ryff (KW 00105206) |
| A. lepidum subsp. haussknechtii | Kos’mo-Damianovskiy Muzhskoy Monastyr (Monastery), Crimea region, Ukraine; in a load-bearing wall | 28.09.2011 | L. Ryff |
| A. ruta-muraria | Sady Górne, Wałbrzych Foothills, Lower Silesia region, Poland; rock crevices | 07.09.2013 | E. Szczęśniak |
| A. ruta-muraria | Near Izborsk in Pskov Governorate and District, Russia; in limestone crevices | 17.08.1899 | W. Andreyev (KW s.n.) |
| A. ruta-muraria | Pokrzywnica, Wielkopolska region, Poland; crevices of an old wall near a large state-owned farm | 01.10.1988 | A. Czarna (POZ-V-0152572) |
| A. ruta-muraria | Tarkhankut Peninsula, Crimea, Ukraine; stony places | 30.07.1974 | O. Dubovik (KW s.n.) |
| A. ruta-muraria | Krobia, Wielkopolska region, Poland; wall crevices in bishop’s palace | 02.09.2021 | Z. Celka |
| A. ruta-muraria | Lubiń, Wielkopolska region, Poland; wall crevices near St Leonard’s church | 18.08.2021 | Z. Celka |
| A. ruta-muraria | Castle on Szczytnik Mt near Szczytna, Lower Silesia region, Poland; castle wall crevices, near rocks | 15.07.2021 | Z. Celka |
| A. ruta-muraria | Czocha Castle, Lower Silesia region, Poland; wall crevice | 05.08.2022 | P. Szkudlarz |
| A. ruta-muraria | Teutonic castle in Toruń, Kujawsko-Pomorskie region, Poland; wall crevice | 25.07.2019 | Z. Celka |
| A. ruta-muraria | Karczówka Monastery, Kielce, Świętokrzyskie region, Poland; crevice of a wall surrounding monastery, shaded site | 17.08.2019 | Z. Celka |
| A. ruta-muraria | Castle in Uzhhorod, Transcarpathian region, Ukraine; wall crevice | 20.09.2018 | Z. Celka |
| A. scolopendrium | Botanical Garden in Wrocław, Lower Silesia region, Poland; ultivated | 10.09.2021 | E. Szczęśniak |
| A. scolopendrium | Village Uholka, Rachiv District, Transcarpathian region, Ukraine; stony places in forest | 14.09.1962 | Herbarium UU (s.n.) |
| A. scolopendrium | Castle in Uzhhorod, Transcarpathian region, Ukraine; in a well | 16.09.2022 | M. Shevera |
| A. scolopendrium | Village Vovchyi… (illegible label) Svaliava District, Transcarpathian region, Ukraine; stony places in forest | 18.07.2011 | O. Kozak (KW 097303) |
| A. scolopendrium | Kąclowa, Małopolska region, Poland; bank of a small stream in fir forest, clay soil | 10.08.1953 | J. Motyka (POZ-V-0004448) |
| A. scolopendrium | In river Punkva valley, Moravský Kras, Czechia; on sides of limestone rocks | 10.1924 | F. Jirasek, J. Podpěra, J. Suza (POZ-V-0004459) |
| A. septentrionale | Cieszów Dolny, Lower Silesia region, Poland; rock crevices | 07.09.2013 | E. Szczęśniak |
| A. septentrionale | Near Ladyzhyn, Vinnytsia region, Ukraine; boh river bank, granite | 19.08.1929 | M. Kotov (KW s.n.) |
| A. septentrionale | S of Fil’akovo, Banská Bystrica region, Slovakia; calcareous slope | 01.07.1956 | F. Celiński (POZ s.n.) |
| A. septentrionale | Village Nezvysko, Obertyn, Iwano-Frankivsk region, Ukraine; rock cracks | 19.07.1940 | Z. Katina (KW s.n.) |
| A. septentrionale | Święty Krzyż, Świętokrzyskie region, Poland; no information | 06.08.1967 | M. Stempel (POZ-V-0152581) |
| A. trichomanes | Castle on Szczytnik Mt near Szczytna, Lower Silesia region, Poland; castle wall crevices near rocks | 15.07.2021 | Z. Celka |
| A. trichomanes | Castle on Szczytnik Mt near Szczytna, Lower Silesia region, Poland; castle wall crevices near rocks | 15.07.2021 | Z. Celka |
| A. trichomanes | Castle in Uzhhorod, Transcarpathian region, Ukraine; wall crevice | 20.09.2018 | Z. Celka |
| A. trichomanes | Uzhhorod, Transcarpathian region, Ukraine; walls | 05.07.2005 | M. Shevera (KW s.n.) |
| A. trichomanes | Castle in Uzhhorod, Transcarpathian region, Ukraine; walls | 16.09.2022 | M. Shevera |
| A. trichomanes | Cieszów Dolny, Lower Silesia region, Poland; rock crevices | 07.09.2013 | E. Szczęśniak |
| A. trichomanes | Jarocin, Wielkopolska region, Poland; wall crevice in an old cemetery | 01.08.1996 | A. Czarna (POZ s.n.) |
| A. trichomanes | Village Bila, bank of Smotrych River, Chemerovets District, Khmelnytskyi region, Ukraine; stone | 23.06.1954 | M. Kotov (KW s.n.) |
| A. trichomanes | Turna on Bodvou, Slovensky Kras, Slovakia; castle ruins, wall crevice | 20.07.2000 | K. Latowski (POZ-V-0152586) |
| A. trichomanes | Village Samchyntsi, Braclav District, Bugrin… (illegible label) Vinnytsia region, Ukraine; rock | 30.08.1932 | N. Osadcha (KW s.n.) |
| A. trichomanes | Miusynsk, near Antratsyt, Luhansk region, Ukraine; no information | 07.06.2013 | M. Peregrym, Ya. Didukh (KW s.n.) |
| A. trichomanes | Gryf Castle, Lower Silesia region, Poland; wall crevice | 06.08.2022 | P. Szkudlarz |
| A. viride | Dolossi (now: Sovietske), Crimea region, Ukraine; rock | 16.07.1956 | M. Kotov (KW s.n.) |
| A. viride | Kvasy, Rachiv District, Transcarpathian region, Ukraine; rock | 18.07.2012 | N. Shyian (KW 001584) |
| A. viride | Trzy Korony Mt (834 m a.s.l.), Pieniny Mts, Małopolska region, Poland; in beech forest | 15.07.1953 | H. Piotrowska (POZ-V-0002171) |
| A. viride | E of Hala Czarnego, Babia Góra National Park, Małopolska region, Poland; On a boulder in stream valley, 1080 m | 25.05.1956 | T. Wojterski (POZ-V-0002166) |
| A. viride | Shipka, Balkans, Bulgaria; no information | 05.05.1976 | W. Żukowski (POZ-V-0002168) |
* – If spores were extracted from herbarium specimens, the collector’s name is followed by the herbarium acronym and sheet number (if given) in brackets. KW – Herbarium of the M. G. Kholodny Institute of Botany in Kyiv; POZ – Herbarium of the Department of Systematic and Environmental Botany (formerly Department of Plant Taxonomy) of Adam Mickiewicz University in Poznań; UU – Herbarium of Uzhhorod National University. s.n. – sine numero.
The names of species and their synonyms follow Hassler (2023).
Description of spore morphology
A random sample of 30 spores from each population was examined. Light microscopy (LM) was used to assess spore size (measurements of polar and longitudinal equatorial diameter), whereas scanning electron microscopy (SEM) was employed to describe microsculpture. Observations and measurements under a light microscope were conducted in an aqueous medium. The measurements took into account the perispore. For polar and longitudinal diameters, the range of values was given (minimum to maximum).
The basic source of biological terms was the Glossary of pollen and spore terminology (Punt et al., 2007), supplemented with terms used in works concerning spores of the genus Asplenium (Ferrarini et al., 1986; Irfan et al., 2022; Puttock & Quinn, 1980; Viane & van Cotthem, 1977; Wei & Dong, 2012). Using the listed sources of morphological terms, we strived to maintain consistency and allow comparisons of our results with the cited publications (so wherever possible, we relied on the same sources as those indicated in the cited works). A detailed analysis of the material with the use of scanning electron microscopy allowed us to describe spore morphology and select diagnostic features (Section 3.2). For their morphological description, taxonomic analysis, and construction of a key to identification, we used the following features of spores, concerning:
major sculptural elements, i.e. folds, which can be: A – low, rib-like (costate type); B – tall with a narrowing crista, i.e. crest (cristate type); C – tall but with a narrow base and nearly straight sides (alate type);
characteristics of fold edges: D – with small protuberances termed micropapillae; E – with larger spines termed echinae, singular echinus (echinate edge); F – with scale-like microsquamae;
presence of pores: G – pores on folds; H – pores between folds;
ornamentation (microsculpture): I – reticulately wrinkled; J – with fine protuberances (micropapillate), K – microsquamate;
surface relief: L – folds cross-linked, forming a network (reticulate); M – folds curved, branched, forming a complicated labyrinth.
Taxonomic analysis
On the basis of the selected diagnostic features presented above (see Section 2.2, Table S1), we developed a key to the identification of the studied taxa on the basis of spore morphology (see Section 3.3) and performed a grouping analysis based on spore similarity (see Section 3.4), with the use of Euclidean distances and cluster analysis: agglomerative clustering. Statistical analyses were carried out using statistical software (Statistica 13.1).
The selected characters are qualitative. To generate the analytical matrix, we treated them as binary characters, coded as 0/1 (absent/ present). These values were recorded for each spore observed under SEM (min. four per sample). Only rarely the number of evaluated spores was lower (immature or damaged spores) (Table S1). Principal component analysis (PCA) was performed to evaluate associations between samples representing the examined species and morphological characters of spores, without any a priori assumptions (Sneath & Sokal, 1973; Stanisz, 2006). The data used for the analysis are presented in Table S1.
. Results
Morphology
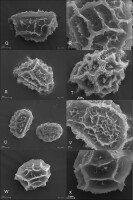
We studied 10 species of the family Aspleniaceae, most of them represented by many samples, in total 57 samples (Table 1). Tetrads in this family are uniplanar (arranged in one plane). The spores are monolete (with one laesura), in various shades of brown, generally ellipsoidal in polar view, reniform to plano-convex in longitudinal equatorial view. Spore size: 25–45 µm × 35–61 µm. Spore sculpture in our study did not vary within species except for A. cuneifolium Viv., A. septentrionale (L.) Hoffm., A. trichomanes L., and A. ruta-muraria L. The group of studied species varied markedly in spore size and surface relief. Out of the 10 studied species, the broadest ranges of variation were observed in A. trichomanes, especially in its spore length, i.e., longitudinal equatorial diameter (Figure 1). Our analysis of this feature reveals the heterogeneity of this species (Figure 2). Sample 6, collected in rock crevices in a forest in the village of Cieszów Dolny in Lower Silesia, is characterized by markedly smaller spores (see Table 1). The folds of perispores were either rib-like, low, with a broad base and no well-defined crista (costate type) (Figure 3N); or tall with a broad base and a well-defined, thin crista (cristate type) (Figure 3D, 3G–H); or tall with a narrow base and nearly straight sides (alate type) (Figure 3Q–R, 3W). Very often, fold edges were not smooth but covered with tiny papillae, less than 1 µm long (micropapillate) (Figure 3Q–R). Less often, fold edges were covered with larger elements – sharp echinae, more than 1 µm long (echinate) (Figure 3G, 3O). Besides, on both fold edges and surfaces, scale-like microsquamae were observed sometimes (Figure 3T). The folds visible on spore surfaces can be cross-linked, with various spaces between them, termed lacunae, forming a reticulate structure (Figure 3B–C) or curved, branched, forming a complicated labyrinth (Figure 3E, 3M). Both the folds and the spaces between them can be smooth (Figure 3Q–R) or ornamented with various types of microsculpture: reticulately wrinkled (Figure 3L), micropapillate (Figure 3B) or microsquamate (Figure 3T, 3V). Microsquamate ornamentation has not been described so far in any Asplenium species. Moreover, in the perispore of most species, pores varying in size and abundance were observed (Figure 3F, 3P, 3X).
Figure 1
Distribution of equatorial diameter (i.e. length) of spores in the studied species of the family Aspleniaceae. 1 – Asplenium adiantum-nigrum; 2 – A. adulterinum; 3 – A. ceterach; 4 – A. cuneifolium; 5 – A. lepidum subsp. haussknechtii; 6 – A. ruta-muraria; 7 – A. scolopendrium; 8 – A. septentrionale; 9 – A. trichomanes; 10 – A. viride.
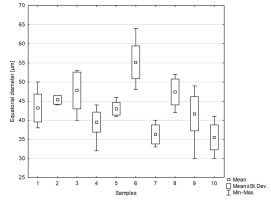
Figure 2
Distribution of equatorial diameter (i.e. length) of individual samples of spores of Asplenium trichomanes. 1–2 – Castle on Szczytnik Mt near Szczytna, Lower Silesia region, Poland; 3 – Castle in Uzhhorod, Transcarpathian region, Ukraine; 4 – Uzhhorod, Transcarpathian region, Ukraine; 5 – Castle in Uzhhorod, Transcarpathian region, Ukraine; 6 – Cieszów Dolny, Lower Silesia region, Poland; 7 – Jarocin, Wielkopolska region, Poland; 8 – Village Bila, bank of Smotrych River, Chemerovets District, Khmelnytskyi region, Ukraine; 9 – Turna on Bodvou, Slovensky Kras, Slovakia; 10 – Village Samchyntsi, Braclav District, Vinnytsia region, Ukraine; 11 – Miusynsk, near Antratsyt, Luhansk region, Ukraine; 12 – Gryf Castle, Lower Silesia region, Poland.
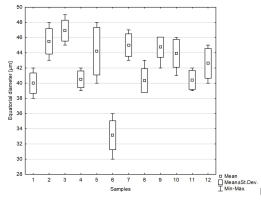
Figure 3
SEM micrographs of fern spores of the studied Asplenium species. A. adiantum-nigrum – distal view (A), surface sculpture (B); A. adulterinum – distal view (C), surface sculpture (D); A. ceterach – distal view (E), surface sculpture (F); A. cuneifolium – distal view (G), surface sculpture (H); A. lepidum subsp. haussknechtii – distal view (I), surface sculpture (J); A. ruta-muraria – distal view (K, M), surface sculpture (L, N); A. scolopendrium – distal view (O), surface sculpture (P); A. septentrionale – distal view (Q), surface sculpture (R); A. trichomanes – distal view (S, U), surface sculpture (T, V); A. viride – distal view (W), surface sculpture (X).


Descriptions of spores of individual species
Asplenium adiantum-nigrum L.
Spore shape prolate in polar view, while D-shaped in longitudinal equatorial view. Spore size: polar diameter 30–35 µm × longitudinal equatorial diameter 40–50 µm. Perispore alate (lophate), with wavy, sharp, narrow ridges (lophae) forming an anastomosing network, sometimes a loose reticulate pattern, rarely forming closed lacunae; fold edges regularly with micropapillae (c. 1 µm). Fenestration: lophofenestrate type, with minute pores (ca. 0.5 µm) located mostly at fold bases. Surfaces between folds varied, often microfolds present. with numerous micropapillae – rugulate/micropapillate ornamentation (Figure 3A–B).
Asplenium adulterinum Milde
Spore shape prolate in polar view, D-shaped in equatorial view. Spore size: polar diameter 33–36 µm × longitudinal equatorial diameter 42–48 µm. Perispore alate (lophate), with wavy, sharp, narrow ridges (lophae) forming an anastomosing network, sometimes a loose network, rarely forming closed lacunae, fold edges irregularly jagged, without regular micropapillae. Fenestration: no pores (imperforate type). Surfaces between folds delicately reticulate, sometimes with fine micropapillae or with lower, irregular ridges (lophae) (Figure 3C–D).
Asplenium ceterach L. (=Ceterach officinarum Willd.)
Spore shape subprolate to prolate in polar view, D-shaped in longitudinal equatorial view. Spore size: polar diameter 35–45 µm × longitudinal equatorial diameter 50–61 µm. Perispore alate (lophate), with wavy, jagged, sharp folds forming an anastomosing network, sometimes a loose reticulate pattern; fold edges with regular micropapillae (ca. 1 µm). Fenestration: pores usually not numerous, present only in spaces between folds (areolofenestrate type). Surfaces between folds smooth as a rule, with infrequent micropapillae, sometimes with ribbed ornamentation (Figure 3E–F).
Asplenium cuneifolium Viv.
Spore shape prolate in polar view, D-shaped in longitudinal equatorial view. Spore size: polar diameter 26–36 µm × longitudinal equatorial diameter 37–45 µm. Perispore cristate (lophate), with wavy, sharp folds forming an anastomosing network, sometimes a loose reticulate pattern; fold edges with regular micropapillae (ca. 1 µm) (serrate type), sometimes with larger spines (to 5 µm, echinate type), Fenestration: usually no pores (imperforate type) but in some samples infrequent, tiny pores at fold bases. Surfaces between folds smooth as a rule, with scattered cone-shaped protuberances micropapillae, sometimes surfaces wavy with papillae – micropapillate type of ornamentation (Figure 3G–H).
Asplenium lepidum C. Presl subsp. haussknechtii (Godet & Reut. ex Milde) Brownsey (=Asplenium haussknechtii Godet & Reut. ex Milde)
Spore shape subprolate to prolate in polar view, D-shaped in longitudinal equatorial view. Spore size: polar diameter 30–35 µm × longitudinal equatorial diameter 41–46 µm. Perispore cristate (lophate), with low, crowded folds, forming a reticulate pattern. Folds ca. 1 µm thick, 1.0–1.5 µm tall. Fold edges with micropapillae, often also larger cone-shaped protuberances, up to 3 µm tall. Fenestration: no pores (imperforate type). Surfaces between folds smooth with fine micropapillae (Figure 3I–J).
Asplenium ruta-muraria L.
Spore shape subprolate to prolate in polar view, D-shaped in longitudinal equatorial view. Spore size: polar diameter 37–48 µm × longitudinal equatorial diameter 50–61 µm. Perispore alate to cristate (lophate), with wavy, sharp, narrow ridges (lophae) forming an anastomosing network, sometimes a loose network, rarely forming closed lacunae. Fold edges vary considerably: in some samples, irregularly jagged, in others, with regular micropapillae. Fenestration: no pores (imperforate type). Surfaces between folds (as well as folds) delicately reticulate, very rarely – in some samples – with fine protuberances micropapillae (Figure 3K–N).
Asplenium scolopendrium L. (=Phyllitis scolopendrium (L.) Newman; =Scolopendrium vulgare Sm.)
Spore shape prolate in polar view, D-shaped in longitudinal equatorial view. Spore size: polar diameter 25–31 µm × longitudinal equatorial diameter 35–43 µm. Perispore cristate (lophate), with wavy, sharp, narrow ridges (lophae) forming an anastomosing network, sometimes a loose network, rarely forming closed lacunae. Folds broad at the base, forming a thin, wing-like crest. Fold edges with regular micropapillae (ca. 1 µm) and often longer outgrowths (some up to 5 µm), either serrate or echinate. Fenestration: lophofenestrate type. Pores 0.5–3.0 µm in diameter, located mostly at fold bases, crowded, so that lophae form an openwork structure. Surfaces between folds often with microfolds – rugulate (ribbed) ornamentation, with numerous fine micropapillae (Figure 3O–P).
Asplenium septentrionale (L.) Hoffm.
Spore shape prolate in polar view, D-shaped in longitudinal equatorial view. Spore size: polar diameter 28–37 µm × longitudinal equatorial diameter 39–48 µm. Spore surface highly variable. Perispore costate to alate, with wavy folds forming an anastomosing network, sometimes a loose reticulate pattern. Folds varying from thick and low (costate) in some samples, to thin and tall (lophae), most frequently. Edges of thick folds (costate) with infrequent micropapillae, but those of thin ones (lophae), usually with crowded micropapillae, sometimes edges echinate. Fenestration: no pores (imperforate type). Surfaces between folds smooth as a rule, rarely with scattered micropapillae (Figure 3Q–R).
Asplenium trichomanes L.
Spore shape subprolate to prolate in polar view, D-shaped in longitudinal equatorial view. Spore size: polar diameter 24–35 µm × longitudinal equatorial diameter 30–48 µm. Spore surface highly variable. Perispore alate to cristate (costate, lophate), with wavy folds forming an anastomosing network, sometimes a loose network, rarely forming closed lacunae. Folds varying from thick and low (costate) to thin and tall (lophate). Fold edges often with scale-like outgrowths (sometimes crowded), rarely echinate. Fenestration: usually no pores (imperforate type), although in a few samples infrequent pores present. Surfaces between folds reticulately wrinkled, forming a delicate network with small outgrowths, scabrate (Figure 3S–V).
Asplenium viride Huds.
Spore shape subprolate to prolate spheroidal in polar view. Spore size: polar diameter 26–35 µm × longitudinal equatorial diameter 30–40 µm. Perispore alate, with straight or slightly wavy, sharp folds, forming a loosely reticulate pattern. Fold edges with regular micropapillae (ca. 1 µm), serrate. Fenestration: pores throughout, infrequent (anomofenestrate type). Surfaces between folds smooth as a rule, sometimes with tiny pores (ca. 0.5 µm) and micropapillae (Figure 3W–X).
Taxonomic analysis
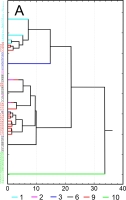
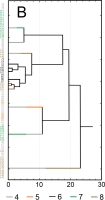
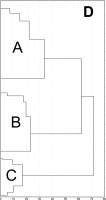
Results of SEM analysis of Asplenium spores indicate that spores of individual species are similar. Detailed morphological characters of the perispore in some species are stable, but in others, they are variable. Because of this, on the basis of the selected features and analysis of the whole material, we attempted to distinguish morphological groups by using statistics, not typology. The attempt was based on agglomerative clustering (Figure 4D). Considering the selected features of the perispore, three morphological groups of spores can be clearly distinguished, although they are not homogeneous. Group A consists of six species, but some of them are markedly distinct in the dendrogram, especially A. viride, A. ceterach as well as A. adiantum-nigrum, which is accompanied by a small group of spores of A. trichomanes (Figure 4A). Besides, this group includes a cluster of A. trichomanes and A. ruta-muraria, which is subdivided into many smaller, mixed clusters. Also A. adulterinum is among them. Group B is composed of five species (Figure 4B), including A. ruta-muraria (observed also in group A). In its two subgroups, spores of A. scolopendrium are located, accompanied by A. cuneifolium. Some subgroups of this heterogeneous group contain A. septentrionale, always accompanied by A. cuneifolium. This group includes also A. lepidum subsp. haussknechtii accompanied by A. cuneifolium, too. Group C is the most homogeneous (Figure 4C). It mostly consists of A. trichomanes, rarely accompanied by A. ruta-muraria, which here forms also a small, separate cluster.
Figure 4
Dendrogram (A–C) of Asplenium spore similarity based on cluster analysis within morphological groups A–C and (D) between these groups. 1 – A. adiantum-nigrum; 2 – A. adulterinum; 3 – A. ceterach; 4 – A. cuneifolium; 5 – A. lepidum subsp. haussknechtii; 6 – A. ruta-muraria; 7 – A. scolopendrium; 8 – A. septentrionale; 9 – A. trichomanes; 10 – A. viride.

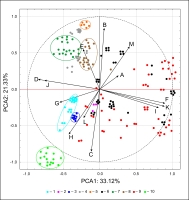
A similar pattern of spore grouping resulted from PCA (Figure 5). The first two principal components carry a total of 54.45% of the variation of the initial variables. The PCA plot presents the clusters of spore samples of Asplenium species. Well-defined clusters of A. scolopendrium, A. septentrionale, A. lepidum subsp. haussknechtii, and A. cuneifolium were distinguished because of their relationship with characteristics of fold edges, E (covered with larger elements classified as echinae, echinate) and with sculptural elements of spore surface, B (tall with a broad base and a conspicuous, thin edge, cristate type). Other homogeneous groups include A. trichomanes, A. ceterach, and A. viride, linked with characteristics of fold edges, G (pores on folds), H (pores between folds), and surface relief, L (folds cross-linked, forming a network, reticulate). A small cluster of A. adulterinum is linked with surface relief, L (folds cross-linked, forming a network, reticulate), with characteristics of fold edges, H (pores between folds), and with sculptural elements of spore surface, C (tall with a narrow base and nearly straight sides, alate type). The remaining samples of A. ruta-muraria and A. trichomanes are highly variable. Some samples of these two species are linked with characteristics of fold edges, F (covered with elements classified as microsquamae), and ornamentation (microsculpture): I (reticulately wrinkled) and K (microsquamate).
Figure 5
Results of principal component analysis (PCA) of interpopulation variation in Asplenium spore diameter. 1 – A. adiantum-nigrum, 2 – A. adulterinum, 3 – A. ceterach, 4 – A. cuneifolium, 5 – A. lepidum subsp. haussknechtii, 6 – A. ruta-muraria, 7 – A. scolopendrium, 8 – A. septentrionale, 9 – A. trichomanes, 10 – A. viride.
Key to species identification based on spore types
Using the analyzed characters of spores (see Section 2.3), we constructed a key to species identification.
1 – Perispore pattern composed of low folds with blunt crests (1 µm wide), forming a dense, net-like pattern; in places where folds branch out, large papillae present (up to 3 µm), while between folds much micropapillae ....................A. lepidum subsp. haussknechtii
1* – Pattern of lophate perispore composed of thin folds (cristate, alate) or thicker, rib-like folds (costate) ....................2
2 – Folds of lophate perispore thin (cristate, alate) ....................3
2* – Folds of perispore rib-like (costate) ....................11
3 – Folds thin, wing-shaped (alate type) ....................4
3* – Folds with a broad base, narrowing into a sharp edge, sometimes with a narrow wing, often edge covered by a row of micropapillae (cristate type) ....................8
4 – Ornamentation (microsculpture) reticulate ....................5
4* – Ornamentation (microsculpture) not reticulate ....................6
5 – Spores >50 µm long ....................A. ruta-muraria
5* – Spores <50 µm long ....................group: A. adulterinum, A. trichomanes
6 – Spores slightly <40 µm long, roundish, folds tall (lophae), often forming regular depressions (lacunae) ....................A. viride
6* – Spores >40 µm long, markedly longer than broad; folds (lophae) forming an irregularly branching labyrinth
7 – Numerous pores at fold bases ....................A. adiantum-nigrum
7* – Pores absent or small, only between folds ....................A. ceterach
8 – Folds strongly porous, like an openwork structure, with pores of various sizes, often >1 µm across ....................A. scolopendrium
8* – Folds with small or no pores ....................9
9 – Ornamentation (microsculpture) reticulate, often also microsquamate ....................10
9* – Ornamentation (microsculpture) not reticulate
10 – Spores >50 µm long ....................A. ruta-muraria
10* – Spores <50 µm long ....................A. trichomanes
11 – Folds and spaces between them sometimes with micropapillae ....................A. septentrionale
11* – Folds and spaces between them densely reticulately wrinkled, sometimes with microsquamae ....................12
12 – Spores >50 µm long ....................A. ruta-muraria
12* – Spores <50 µm long ....................A. trichomanes
** group: A. adulterinum, A. trichomanes.A. adulterinum – as a rule, folds with micropapillae and no microsquamae. A. trichomanes ornamentation microsquamate. These features are unstable and poorly distinguish between the two taxa.
*** group: A. cuneifolium, A. septentrionale. A. cuneifolium spores ca. 35–43 µm long, folds narrowing into a thin crest, often with no wing, sometimes with a narrow, interrupted wing; edges with micropapillae, sometimes sharp outgrowths (echinae). A. septentrionale spores ca. 43–48 µm long, micropapillae on folds or between them, no echinae. These features are unstable and poorly distinguished between the two taxa.
. Discussion
Spores of all the 10 studied species of the family Aspleniaceae are of one basic type: uniplanar, monolete, prolate (or subprolate) in equatorial longitudinal view. This is consistent with earlier reports on this subject (e.g., Curto et al., 2012; Irfan et al., 2022; Kramer & Viane, 1990; Mazooji & Salimpour, 2014; Pangua & Prada, 1988; Regalado & Sánchez, 2002; Wei & Dong, 2012). This raises doubts about Lashin’s (2012) report about trilete spores in the genera Asplenium and Ceterach. Most probably, his observations refer to contamination or the trilete spores reported that came from a sample erroneously identified as Asplenium or Ceterach.
The number of species included in this study and the number of analyzed samples allow us to draw both general and detailed conclusions. They are generally consistent with the conclusions of many researchers who studied spore morphology in the family Aspleniaceae. This applies to the high variation of perispore structure within the genus and simultaneously remarkable stability of spore morphology within species (Irfan et al., 2022; Pangua & Prada, 1988; Wei & Dong, 2012). As emphasized by the cited authors, this makes it possible to use morphological features of spores for the identification of at least some species. Our results confirm that high stability of perispore morphology is observed in A. adiantum-nigrum, A. ceterach, A. lepidum subsp. haussknechtii, and A. viride. The distinctness of spore morphology in these species is reflected by the results of both agglomerative clustering (Figure 4) and PCA (Figure 5). This group includes also A. scolopendrium, clearly distinct from the other studied species (Figure 3O–P). In this analysis, spores of this species form two clusters (Figure 4B), because some spores were classified as echinate, but this does not change the generally high morphological homogeneity of spores in this taxon, illustrated by the results of PCA (Figure 5). The above-mentioned feature may indicate some regional differences, which distinguish the southern population located in mountainous regions (Moravia in Czechia and Transcarpathia in Ukraine). Considering A. ceterach, pores in the perispores were found in the examined samples, but absent in samples from Pakistan (Irfan et al., 2022).
A great variation in spore morphology was observed in species like A. trichomanes and A. ruta-muraria (Figures 4 and 5). Both species have reticulate perispores, in a majority of samples with microsquamate ornamentation (microsquamae covering more or less densely both the folds and the spaces between them). Such an ornamentation has not been described so far in any Asplenium species, although a similar ornamentation is visible on micrographs in an article by Redondo et al. (1999). In A. trichomanes, the perispore can be devoid of any perforation, but in some samples, pores are present. In this species, pores were observed in samples with smaller spores, which may belong to diploid forms, as noted by Pangua and Prada (1988). A. ruta-muraria has highly variable folds, which can be classified as costate, cristate, or alate types. A high variability of spores was also observed in A. cuneifolium. Although all its samples are in group B (Figure 4B), they are scattered in various clusters. The variation in perispore morphology of this species is affected by two features: the presence of pores (pores are present but not in all samples) and the presence of echinae on folds. Spores of A. septentrionale seem to be homogeneous, but in samples from Poland, folds of the perispore are rib-like, costate, while in the others, they are cristate.
Most of the examined spores, with respect to their length, have stable ranges of variation for most of the studied species. Only within A. trichomanes some samples contain markedly smaller spores. This may be linked with ploidy (see Ferrarini et al., 1986; Pangua & Prada, 1988). In comparison with spore length ranges listed by other authors (Bobrov et al., 1983; Ferrarini et al., 1986; Lin & Viane, 2013; Mazooji & Salimpour, 2014; Reichstein, 1984; Shah et al., 2020), the data are similar in most cases. Remarkable differences concern A. ceterach, as a majority of researchers reported much smaller values than those recorded in our study. A similar size range was given only by Bobrov et al. (1983). Also, in the case of A. trichomanes, only Bobrov et al. (1983) reported a similar size range, while the other authors presented relatively low values, close to only a few samples in our study. The observed variation in spore size of some species may result from polyploidy, which has been reported many times (e.g., Barrington et al., 1986; Ekrt & Štech, 2008; Ferrarini et al., 1986; Pangua & Prada, 1988).
The described spore morphology and the open grouping based on the morphological characteristics of spores are not consistent with the results of an earlier phylogenetic analysis (Xu et al., 2020). The studied species belong to four separate clades. However, phylogenetically closely related taxa differ in perispore morphology, so they form completely different groups. Interestingly, the species with variable spore morphology also show variability in phylogenetic analysis (e.g., A. ruta-muraria or A. trichomanes).
In brief, this study provides detailed descriptions of spores of Central and Eastern European fern species of the family Aspleniaceae. It includes the first detailed description and micrographs of spores of a new species for the flora of Eastern Europe, discovered in 2011: A. lepidum subsp. haussknechtii (Ryff, 2013). For earlier described species (A. ceterach, A. trichomanes), our results supplement knowledge about spore variation (e.g., Shah et al., 2020). Our results show a need for more detailed research on spore size in relation to ploidy, but it requires the use of more material.


