Introduction
Research on the moss flora in the vast expanse of mainland sub-Saharan Africa began relatively late, only in the second half of the nineteenth century, with the progressive exploration and discovery of areas unknown to Europeans (Büttner,1889, pp. 67–68; Mitten,1860,1863,1886; Müller,1875,1876,1879,1888,1890,1893). The only exception to this rule was Cape Province in South Africa, whose modern history began in 1488 with the rounding of the Cape of Good Hope by Bartolomeu Dias. The strategic location of this area on the main trade route between Europe and the West Indies meant that Cape Town, founded in 1652 by the employees of the Dutch East India Company, became the transoceanic port city, gaining a reputation over time as “the tavern of the seas.” The magnet attracting horticulturists and botanists was the extremely rich and diverse flora of the Cape region, which was soon recognized as a separate plant kingdom, Capensis.
The first collection of bryophytes in South Africa was made by Carl Pehr Thunberg (1743–1828), a Swedish naturalist and pupil of Linnaeus, who recorded five hepatic and 14 moss species from the Cape (Thunberg,1800). He was the first professional botanist who personally collected extensively in South Africa between 1772–1775 and laid the foundation for taxonomic botany in this country, thereby gaining the name “father of South African botany.” Thunberg was soon followed by others who collected bryophytes as members of expeditions of discovery, such as A. Menzies and R. Brown, and people who landed here for professional activities, such as W. J. Burchell, C. H. Bergius, Ch. F. Ecklon, and C. L. Zeyher, or professional plant collectors, including J. L. Mund, J. F. Drège, L. Maire, and Ch. F. von Krauss (Glen & Germishuizen,2010). In an overwhelming majority of cases, the specimens they collected were passed on to researchers in Europe, who, as a rule, described them as new species for science (e.g., Hooker,1818–1820; Hornschuch,1841; Krauss,1846, pp. 132–134). Only in exceptional cases did the collectors themselves describe the new species (e.g., Brown,1819; Harvey,1838; Harvey & Hooker,1836). All early records, up to the mid-1870s, were summarized by Shaw (1878) in a catalog of the mosses of the Cape Colony.
The next half-century brought further significant progress to the study of the moss flora of South Africa. The Polish botanist, A. Rehmann (1840–1917), was of particular importance in this field (Codd & Gunn,1982). During his two research trips to this country, he made a significant collection of mosses, which he distributed in two series of the exsiccata Musci Austro-Africani, consisting of 680 numbers (Dixon & Gepp,1923). They were examined by Müller (1888,1899) who described many specimens as new species. This period in the history of bryological studies in southern Africa was summarized by Sim (1926) in his bryophyte flora of this region, in which he recognized 490 species of moss.
After another 53 years, a newly updated checklist of the mosses of southern Africa, comprising the countries of South Africa, Namibia, Botswana, eSwatini, and Lesotho, was published, covering 591 species (Magill & Schelpe,1979). Additionally, it was to provide a basis for a forthcoming descriptive flora of mosses of this region as part of the “Flora of southern Africa” project. During the 19 years that followed, three of four planned volumes of this work were published (Magill,1981,1987; Magill & Van Rooy,1998), but unfortunately, this project has never been completed. In the most recent checklist of southern African mosses, 546 species and nine infraspecific taxa have been recorded from the region (Van Rooy,2003).
Material and Methods
On a collecting trip to the Limpopo Province of South Africa in the year 2000, the late Sarie M. Perold (PRE; Van Rooy,2012) collected a specimen of Ephemerum Hampe that did not fit any of the known species of the genus in southern Africa. It was compared with other species of Ephemerum and matched the South American endemic E. homomallum Müll. Hal., thus a new record for the region.
During a revision of southern African Brachytheciaceae, a specimen from Hogsback in the Eastern Cape Province of South Africa, collected by Miss Young in 1926, was identified as Torrentaria aquatica (A. Jaeger) Ochyra. These two species have thus far been known only from the Neotropics and are reported for the first time from Africa, thereby exhibiting an Afro-American distribution pattern.
The specimens supporting this study are deposited at PRE.
Results
Ephemerum homomallum Müll. Hal.
Flora 71: 12. 1888. Type citation: Paraguay, summitate montis Cerro de Yaguaron (sic!) supra terram, 17. Junio 1879: B. Balansa, Coll. No. 2621.
Specimen Examined
AFRICA. South Africa: Limpopo Province, Polokwane (Pietersburg) Nature Reserve, 23°55′39″ N, 29°27′48″ E, alt. ca 1,275 m a.s.l., on damp soil near road, May 1, 2000, Perold 4389 (PRE).
Distribution
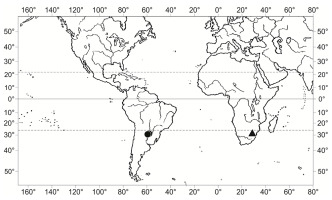
Ephemerum homomallum is one of the rarest species of the genus Ephemerum Hampe, which has thus far only been known from two collections from Paraguay (Müller,1888; O’Shea & Price,2008; Schiavone & de Sarmiento,1985). They are situated in Cerro de Yaguarón (25°34′15″ N, 57°17′39″ W, alt. ca. 255 m a.s.l.) and in the nearby village of Guarapi (25°34′07″ N, 57°15′07″ W, alt. ca. 230 m a.s.l.), approximately 50 km southeast of Asunción in the Yaguarón District in Departamento de Paraguarí. Therefore, for over 110 years, E. homomallum was accepted as an endemic of Paraguay (O’Shea & Price,2008). The present discovery in South Africa established E. homomallum as an Afro-American disjunct moss species (Figure 1). It was discovered in the Polokwane (Pietersburg) Game Reserve, near the Limpopo capital of Polokwane, which conserves the Polokwane Plateau Bushveld, a threatened vegetation type (Mucina & Rutherford,2006). This locality falls within the Zambezian Bryofloristic Region sensu Van Rooy and Van Wyk (2010), which covers bushveld or savanna areas in the northern part of southern Africa.
Remarks
Ephemerum is a genus consisting of pygmy and, as the name suggests, ephemeral, scattered or gregarious mosses, which very often arise from persistent copiose protonemal mats. They are pioneers of bare disturbed soils in temporarily available sites, growing most often on fallow ground, silty substrate in floodplains, and along the margins of ponds, lakes, pools, and streams. Owing to its minute size, as well as a short life cycle and a seasonal habitat, species of Ephemerum are usually overlooked in the field. This results in a paucity of collections of these mosses in herbaria and accordingly, they are considered rare or very rare, when in fact this could be an artifact of their under-collection.
Species of Ephemerum are distributed worldwide, although they are mostly found in the temperate regions of both hemispheres, they are rare and localized in the tropics, and absent from the polar regions. The genus consists of 20 species, which are now generally accepted, although nine species need a careful taxonomic assessment because they have not been studied since their descriptions (Crosby et al.,2000; Ellis & Price,2015; Frey & Stech,2009; Holyoak,2010). Traditionally, Ephemerum was positioned in a family of its own, Ephemeraceae, but molecular data established that it is nested in the Pottiaceae subfamily Trichostomoideae (Goffinet & Cox,2000), although morphologically it appears to be a discordant element in this family.
The genus Ephemerum was revised for the moss flora of southern Africa by Magill (1987), who recognized four species [E. capense Müll. Hal., E. rehmannii (Müll. Hal.) Broth., E. diversifolium Mitt, and E. namaquense Magill], of which the last two are endemic to the region. The same four species are accepted in the most recent checklist of South African mosses (Van Rooy,2006). It is worth noting that Shaw (1878) described E. piliferum J. Shaw from the Cape, but the status of this species has not been resolved because of the lack of type material and an inadequate description (Magill,1987; Sim,1926). In sub-Saharan Africa, two additional species of Ephemerum have been recorded, namely E. perminutum C. C. Towns. from Tanzania (Townsend,1981) and E. pechuelii Müll. Hal. from the Central African Republic, Nigeria, and the Democratic Republic of the Congo (O’Shea,2006).
Ephemerum homomallum was originally described by Müller (1888) from material collected in the southwestern part of Paraguay, close to the Argentinian border. In a revision of the genus Ephemerum for Argentina, Schiavone and de Sarmiento (1985) provided nomenclatural information, descriptions, illustrations, and a key to the four species recognized in the moss flora of Argentina and adjacent territories, including E. homomallum. In an updated checklist of the mosses of Paraguay (O’Shea & Price,2008), E. homomallum is accepted as an endemic to the country.
Gametophytically, Ephemerum homomallum differs from the closely related E. capense Müll. Hal. in having a more distinct and abundant protonema, fewer leaves that are shorter and narrower, thicker walls in cells above the middle and at the position the costa would occupy, lacking sharp spines on the margin, and having a smoother calyptra wall. Additionally, it differs from all other relatives in southern Africa in having the largest spores that are ellipsoid, 88 μm long, and 62 μm wide.
Torrentaria aquatica (A. Jaeger) Ochyra
Nova Hedwigia 96(1–2): 210. 20 Nov 2012 ≡ Hypnum aquaticum Hampe, Linnaea 32: 61. 1863, hom. illeg. ≡ Rhynchostegium aquaticum A. Jaeger, Ber. Thät. St. Gall. Naturw. Ges. 1876–1877: 378. 1878 ≡ Oxyrrhynchium aquaticum (A. Jaeger) Broth. in Engl. & Prantl, Nat. Pflanzenfam. 1(3): 1155. 1909 ≡ Platyhypnidium aquaticum (A. Jaeger) M. Fleisch., Musci Buitenzorg 4: 1537. 1923. Type citation: [Nova Granada] Bogota Los Laches in rivulis ad saxa, 2800 metr., Junio [Lindig] no. 2024.
Specimen Examined
AFRICA. South Africa: Eastern Cape Province, near Tyumie Fall, Hogsback, 32°40′30″ N, 26°48′15″ E, alt. ca. 1,160 m a.s.l., on rocks in rushing water, December 26, 1926, Young sub Sim 3005 (PRE).
Distribution
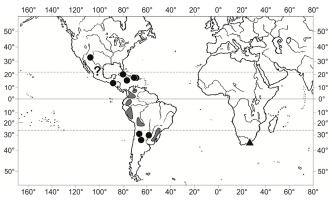
Owing to unresolved taxonomic problems, the phytogeographical status of Torrentaria aquatica, a species better known as Platyhypnidium aquaticum, is difficult to determine. Herein, it is traditionally interpreted as a neotropical species, occurring in the Caribbean (Cuba, Jamaica, Hispaniola) (Buck,1998; Duarte Bello,1997) and some Central American countries, including Guatemala, El Salvador, Honduras, Costa Rica, and Panama (Allen,2018), and in the North and Central Andean countries (Venezuela, Colombia, Ecuador, Peru, Bolivia) in South America (Churchill et al.,2000) extending to southeastern Brazil (Yano,1981) and northern Argentina (Matteri,2003). Additionally, Allen (2018) cited a single specimen of this species from New Mexico in the south-central United States and reported it from Mexico but without providing specific locations and T. aquatica had not been recorded from this country before (McFarland,1994).
Herein, Torrentaria aquatica is established as an amphiatlantic species following its discovery in the Eastern Cape Province in South Africa (Figure 2). The voucher specimen was collected in 1926 by Miss Young and sent to T. R. Sim who identified it as Amblystegium riparium (Hedw.) Schimp. var. rivulare Dixon & A. Gepp, a varietal name that has not been validly published. The Hogsback locality falls within the Afromontane Bryofloristic Region of Van Rooy and Van Wyk (2010), characterized by indigenous forest patches in grasslands or fynbos and the abundance of pleurocarpous mosses. The Tyume Falls is situated in a stream running through indigenous forest, approximately 1,160 m a.s.l.
Remarks
The generic position of the species in question has long been the subject of controversy and discussion. It has been interchangeably placed either in the genus Rhynchostegium Schimp. or Platyhypnidium M. Fleisch. in Brachytheciaceae. The latter genus has been also placed in the Amblystegiaceae by some authors because of the aquatic preference of its species and the overall similarity to some species of Hygrohypnum Lindb. As the generic name Platyhypnidium is illegitimate, Ochyra (2013) proposed the name Torrentaria Ochyra for a group of species from the Brachytheciaceae having sparingly branched stems and very broad, monomorphic leaves with rounded-acute to obtuse apices and serrate to serrulate margins all around, enlarged cells across the basal insertion region, and weakly developed angular cells. Species of this genus are rheophytes usually growing on rock in waterfalls or streams with swiftly flowing water and exhibit some morphological and anatomical adaptations to such habitats, including broad, salient, and often spurred costae and variously polystratose laminal cells (Ochyra,1985,1985,1986,1987; Ochyra et al.,1998; Ochyra & Vanderpoorten,1999).
The core of the genus Torrentaria constitutes a group of species centered around the Holarctic T. riparioides (Hedw.) Ochyra, with T. aquatica, which is widespread in the New World, and T. muelleri (A. Jaeger) Ochyra & Bedn.-Ochyra, which is its Asian counterpart (Ignatov et al.,1999; Ochyra & Bednarek-Ochyra,2014). Similar to other aquatics, they exhibit significant plasticity, and generally, they are morphologically very similar. The former two are best distinguished only by subtle differences in the shape of their alar cells and details of the peristome teeth. In T. aquatica, the alar cells are thin-walled, oblong to broadly-rectangular, and form an enlarged and usually bulging group beyond the leaf margins. In contrast, the alar cells of T. riparioides are firm-walled, subquadrate to subrectangular, only slightly enlarged, and form a plane or faintly bulging group beyond the leaf margins. Additionally, the cilia of T. aquatica are short, reaching only half the segment length, whereas in T. riparioides they are longer, up to two-thirds or three-quarters of the segment length.
The distinctness of these species was confirmed by molecular data (Wagner et al.,2005). Additionally, the molecular phylogenetic analyses demonstrated that the North American and European populations of Torrentaria riparioides are genetically different (Huttunen et al.,2007; Huttunen & Ignatov,2010). Accordingly, despite the lack of any morphological diagnostic characters, North American plants, which had thus far been considered T. riparioides, were recognized as an endemic species, Rhynchostegium shawii Hutsemékers & Vanderp. (Hutsemékers et al.,2012). Alternatively, Ignatov (2014) included all North American plants of T. riparioides into T. aquatica, despite some morphological differences between the two species.
It is very likely that Torrentaria aquatica is a pantropical species if the problematic American–East Asiatic and Malesian–Australian groups of populations, recognized by molecular data (Huttunen & Ignatov,2010) are combined into a single species. The plants from New South Wales in southeastern Australia were so-named (Hedenäs,2012 as Platyhypnidium aquaticum) and the East Asiatic and Malesian P. muelleri was considered conspecific with it, although without a formal typification of the species names concerned. Similarly, Allen (2018) was strongly inclined to accept the conspecificity of these species after comparison of the neotropical and Javanese plants.
An open question is the status of East African populations of Torrentaria, which are recognized as T. riparioides and T. hedbergii (P. de la Varde) Ochyra & Sharp (Ochyra & Sharp,1988). It is highly likely that they truly form a separate Afro-European group represented by T. riparioides (Huttunen & Ignatov,2010), genetically distinct from the American-Asiatic-Australian group. The deep penetration of the Holarctic moss species in the East African mountains is well documented (e.g., Bizot & Pócs,1974; Koponen,1993; Ochyra & Bednarek-Ochyra,2000,2002; Ochyra, Wesche, et al.,2002). However, it does not preclude the occurrence in Africa of neotropical or pantropical species and southern Africa yields quite a large number of examples of this distribution pattern (e.g., Ochyra et al.,2013,2015; Ochyra & Newton,1985; Ochyra & Van Rooy,2013).
Discussion
As the taxonomy of African and Neotropical bryophytes has become better known and the exploration of understudied areas has progressed, more species are increasingly found to occur on both continents. These are mostly lowland and montane tropical mosses (e.g., Arts,1998; Bednarek-Ochyra et al.,1999; Buck & Griffin,1984; Ellis et al.,2013,2014,2016; Ochyra et al.,1992; Ochyra & Ireland,2016; Orbán,2000; Porley & Edwards,2010; Shaw et al.,2008). This type of distribution is also exhibited by a small group of austral cool-adapted mosses, which occur in the temperate regions of southern South America and extend to southern Africa and African islands in the Southern Ocean, or both (e.g., Bednarek-Ochyra,2014; Bednarek-Ochyra et al.,1996; Bednarek-Ochyra & Ochyra,1998,2012,2012; Blockeel et al.,2009,2010; Li et al.,2009; Ochyra,2010; Ochyra & Bednarek-Ochyra,2013; Ochyra, Bednarek-Ochyra, & Lewis Smith,2002; Ochyra & Bell,1984; Ochyra & Lewis Smith,1998; Ochyra, Lewis Smith, & Bednarek-Ochyra,2008; Ochyra & Lightowlers,1988).
Of the bryophytes, only liverworts were surveyed in detail in the Afro-American element (Gradstein,2013; Gradstein et al.,1983) and currently, this group is represented by 78 species. Afro-American disjunct mosses have been compiled by Delgadillo (1993), but it is a list of all species that the Americas share with Africa, not species found exclusively on both continents and not recorded elsewhere. The tentative unpublished list of such mosses consists of over 80 species, which are common to both continents (Ochyra & Plášek,2019). This paper reports two additional moss species that exhibit an Afro-American distribution pattern. They are new to South Africa, as well as to continental Africa.
The moss flora of South Africa is not only the best known in Africa but also the Southern Hemisphere. In the latest checklist of mosses, 540 species and infraspecific taxa have been recorded from this country (Van Rooy,2006). Since then, this number has increased by approximately 30 species. Nine of these represent species newly described to science based upon discoveries made during recent field studies. These are Triquetrella mxiwana Hedd. & R. H. Zander (Zander & Hedderson,2007), Chenia ruigtevleia Hedd. & R. H. Zander (Hedderson & Zander,2008), Bryobartramia schelpei Hedd. (Hedderson,2012), Sematophyllum magillianum P. E. A. S. Câmara & Van Rooy (Câmara & Van Rooy,2014), Ludorugbya springbokorum R. H. Zander & Hedd. (Zander & Hedderson,2007), Algaria nataliei R. H. Zander & Hedd. (Zander & Hedderson,2008), Acaulonopsis eureka R. H. Zander & Hedd., A. fynbosesnsis R. H. Zander & Hedd. (Zander & Hedderson,2009), and Picobryum atomicum R. H. Zander & Hedd. (Zander & Hedderson,2011). Additionally, Vrolijkheidia circumscissa Hedd. & R. H. Zander was described as a new species from the Western Cape Province (Hedderson & Zander,2008), but it proved to be a species described much earlier as Phascum peraristatum Müll. Hal. (Müller,1888); therefore, the correct name being Vrolijkheidia peraristata (Müll. Hal.) R. H. Zander & Hedd. (Zander & Hedderson,2009). It is worth noting that the last five genera are endemic to South Africa. Apart from these nine new species and five new genera for South Africa, an additional new species, Andreaea barbarae Luceño, C. Cerrejón, J. Muñoz & Maguilla, was recently discovered in Lesotho (Cerrejón et al.,2018). All these records of new species and genera indicate that, despite a long history of bryological exploration, southern Africa still holds remarkable undiscovered biodiversity, including many unique taxa of considerable evolutionary and biogeographical importance.
Apart from the aforementioned newly described species, an additional 19 species have been newly recorded in South Africa since the 2006 checklist (Van Rooy,2006), including those presented in this account, which represent various geographical elements. Some are Afro-American disjuncts, namely Bucklandiella striatipila (Cardot) Bedn.-Ochyra & Ochyra (Bednarek-Ochyra & Ochyra,2010,2013), Isopterygium tenerifolium Mitt. (Ochyra & Ireland,2016), Guembelia kidderi (James) Ochyra & Żarnowiec (Maier et al.,2017), and Didymodon fuscus (Müll. Hal.) J. A. Jiménez & M. J. Cano (Hedderson & Jiménez,2017).
The discovery of Plagiothecium lamprostachys (Hampe) A. Jaeger (Blockeel et al.,2005), Bucklandiella didyma (Mont.) Bedn.-Ochyra & Ochyra (Ochyra, Bednarek-Ochyra, & Lewis Smith,2008), Dicranella hookeri (Müll. Hal.) Cardot (Ochyra et al.,2013), Dryptodon tortuosus (Hook. f. & Wilson) Ochyra & Żarnowiec, Grimmia consobrina Müll. Hal., and G. pygmaea Müll. Hal. (Maier et al.,2017) confirm the strong affinities of the South African moss flora with other Southern Hemisphere continents. Moreover, the occurrence of Neckera complanata (Hedw.) Huebener (Blockeel et al.,2006), Orthogrimmia montana (Bruch & Schimp.) Ochyra & Żarnowiec, Dryptodon orbicularis (Wilson) Ochyra & Żarnowiec (Maier et al.,2017), and Bucklandiella sudetica (Funck) Bedn.-Ochyra & Ochyra (Bednarek-Ochyra, 2018) indicate the deep penetration of Holarctic oreophytes into the tropics along the African track.
The diversity of the various floristic elements in the moss flora of South Africa is complemented by two pantropical species, Syntrichia amphidiacea (Müll. Hal.) R. H. Zander (Blockeel et al.,2001) and Isopterygium tenerum (Sw.) Mitt. (Ochyra & Ireland,2004), and the African tropical species Hylocomiopsis cylindricarpa Thér. (Ellis et al.,2011). To conclude, it should be noted that the southern African moss flora has been enriched by another species, Leptodontium proliferum Herzog (Porley & Edwards,2010), that was found in Lesotho.
Unfortunately, additions to the moss flora of South Africa are partially diminished by species that must be excluded from the flora as incorrectly reported. This applies to, among others, Sematophyllum wageri C. H. Wright ex Wager and S. zuluense (Sim) Magill, which proved to be conspecific with S. brachycarpum (Hampe) Broth. (Câmara et al.,2019), as well as Guembelia ovalis (Hedw.) Müll. Hal., and Dryptodon trichophyllus (Grev.) Brid., whose voucher specimens represented different species of this difficult genus (Maier et al.,2017). Likewise, Bucklandiella crispula (Hook. f. & Wilson) Bedn.-Ochyra & Ochyra, which was recorded from South Africa as Racomitrium crispulum (Hook. f. & Wilson) Wilson (Bednarek-Ochyra,2015; Magill,1981), was very broadly interpreted (Clifford,1955) and the species in a strict sense is a narrow endemic of the Campbell and Auckland Islands, south of the South Island of New Zealand in the Southern Ocean (Bednarek-Ochyra & Ochyra,2011; Bednarek-Ochyra et al.,2014).
Handling Editor
Beata Zagórska-Marek; University of Wrocław, Poland; https://orcid.org/0000-0001-6385-858X