Introduction
Detailed knowledge of the flora of any area is crucial to recognize patterns and understanding the processes affecting biodiversity as well as to point out the ways of its conservation, both at a local and global scale (Gaujour et al., 2012). Being under increasing pressure from human activities and recently also under the negative impact of global climate warming, the dynamics of both flora and vegetation in the last decades have been much faster than they used to be (Araújo & Rahbek, 2006; Bellard et al., 2012). These changes are reflected in the progressing decline of the species sensitive to environmental changes as well as in the spread of invasive alien species, negatively affecting natural ecosystems and their services important for human well-being. Information on the occurrence of species in areas previously not occupied by them, unnoticed or misidentified with other species, is of great interest and continues to be published (e.g., Bulakh et al., 2022; Dudáš et al., 2022; Ellis et al., 2022; Nobis et al., 2019b; Raab-Straube von & Raus, 2022; Tlałka et al., 2021; Verkhozina et al., 2022) since each new floristic record broadens our knowledge of the species spatiotemporal dynamics and contributes to more effective management of natural resources.
This paper is a continuation of the previous works (e.g., Nobis et al., 2014, 2018, 2019b, 2019c) and is dedicated to new findings on the distribution and taxonomy of vascular plants, or simpler, to plants new to the flora of selected countries (or their significant regions) in the Old Word (including Europe, Asia, and Africa). Here, we present the data on 16 species that are newly reported as components of the flora of five countries or their significant regions (provinces or republics) – five taxa are given for the first time from Poland, and one is rediscovered and thus reassessed, five are new records to Tajikistan, three to Kyrgyzstan, one to Turkey, one to China and one to the Gansu Province in China. The taxa listed below are given alphabetically.
Achnatherum jacquemontii (Jaub. & Spach.) P.C. Kuo & S.L. Lu (Poaceae)
Synonyms: Stipa jacquemontii Jaub. & Spach.; Lasiagrostis jacquemontii (Jaub. & Spach.) Munro ex Boiss; Lasiagrostis jacquemontii (Jaub. & Spach.) Munro ex Aitch.; Achnatherum botschantzevii Tzvel.; Stipa jacquemontii Jaub. & Spach. subsp. chuzomica Noltie.
Contributors: Marcin Nobis, Ewelina Klichowska
New record
TAJIKISTAN: Kyzylsuu River Valley, Achikalma settl. ca. 24 km ENE of Damburacha, 39°22′9.34″N / 71°38′39.14″E, elevation 2105 m; exp. S-SW, incl. 50–80%, 16 Jul 2021, wp. 1476, M. Nobis, E. Klichowska s.n. (KRA 594552-594557, 594559, 594560)
Taxonomic notes
The genus Achnatherum in Tajikistan comprises three species so far: A. caragana, A. turkomanicum, and A. splendens. The latter, based on distinct macro- and micromorphological characters, has recently been transferred to the genus Neotrinia as N. splendens (Trin.) M. Nobis, P.D. Gudkova & A. Nowak (Nobis et al., 2019b).
Newly reported from Tajikistan, Achnatherum jacquemontii is well distinguished from all the other above-mentioned taxa from the genus (Figure 1) by having awns 20–35 mm long, clearly unequal lemma and palea, filiform and convolute leaves, culms up to 45 cm long with panicles having short branches (Freitag, 1985; Nobis et al., 2019a).
Figure 1
Achnatherum jacquemontii (A) in Kyzyl-Suu River Valley near Achikalma settl. in Tajikistan (photo. M. Nobis, 2021); (B) cluster analysis (UPGMA based on Gower’s general similarity coefficient, see Supplementary file) performed on eight quantitative and two qualitative characters of the Central Asian representatives of the genus Achnatherum.
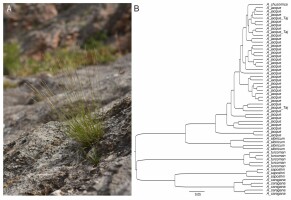
Distribution and habitat
Achnatherum jacquemontii is a south-central Asian species that occurs in Kyrgyzstan, Uzbekistan, Tajikistan, Afghanistan, Pakistan, Nepal, India, China, and Bhutan (Freitag, 1985; Nobis et al., 2019a, 2020; Wu & Phillips, 2006). In the mountains of Central Asia, A. jacquemontii is distributed in Alai and Turkestanian Mts (Nobis et al., 2016), and these are the northernmost localities of the species within its distribution range. In Tajikistan, the species grows on calcareous rocks in the northern part of the country (Figure 1). It is a native species in the flora of Tajikistan and the main component of the Asperulo oppositifoliae-Achnatheretum jacquemontii plant associations which occurs in rocky crevices and shelves on steep rocky walls, especially within the rocky breakthroughs of several river valleys. The association was noted in Isfara, Sokh, Kyshtut, Taraty, and Kyzyl-Suu River Valleys in Kyrgyzstan and Tajikistan. Patches of this association were found in the montane and subalpine zones of both the Alai and Turkestan Mts, mainly at the elevation of 1600–2200 m. The association of Asperulo oppositifoliae-Achnatheretum jacquemontii develops on shallow alkaline soils in rocky crevices and shelves on different types of calcareous rocks. Within the examined plots, apart from A. jacquemontii as a dominant species, Asperula oppositifolia and Nepeta subhastata have relatively high constancy in the association, as well as some other species typical for rocky substrates in this region and passing from the other associations, belonging to the Campanuletalia incanescentis order, e.g., Campanula incanescens, Scutellaria immaculata, or Pentanema albertoregelia. The patches of Asperulo oppositifoliae-Achnatheretum jacquemontii are relatively poor in species (Table S1 in the Supplementary material), which is generally typical for chasmophytic vegetation (Nobis et al., 2013; Nowak et al., 2022). The syntaxonomic position of the newly described associations is as follows:
Class: Asplenietea trichomanis (Br.-Bl. in Meier & Br.-Bl. 1934) Oberd. 1977
Order: Campanuletalia incanescentis M. Nobis, A. Nowak & A. Nobis 2013
Alliance: Asperulo albiflorae – Poion relaxae M. Nobis, A. Nowak & A. Nobis 2013
Association: Asperulo oppositifoliae-Achnatheretum jacquemontii M. Nobis, Klichowska & A. Nowak 2023, ass. nov., hoc loco.
Type relevé (holotypus): Kyrgyzstan, Alai Mts, S of Sytr settl., left slope of Tarty River valley, calcareous rocks, slope 85%, inclination SSE, 39°52′25″N / 71°16′02″E, elev. 2030 m; area of rel. 1.7 m2, 1 Jul 2016, cover of herb layer ‘C’ 30%: Achnatherum jacquemontii 2, Artemisia rutifolia 1, Asperula oppositifolia +, Campanula incanescens +, Nepeta subhastata +, Pentanema albertoregelia +, Spiraea pilosa +.
Achnatherum sibiricum (L.) Keng ex Tzvelev (Poaceae)
Synonyms: Avena sibirica L.; Stipa sibirica (L.) Lam.; Stipa avenoides Honda; Achnatherum avenoides (Honda) Y. L. Chang
Conributors: Marcin Nobis, Agnieszka Nobis
New record
KYRGYZSTAN: Issyk-kul Region, ca. 64 km to the SW from Balykchy (on the W edge of Issyk-Kul Lake), and ca. 23 km to the SW from Kochkor village, near the road A365, near rocks, 41°59′25.72″N / 75°42′58.80″E, elev. 2218 m, wp. 825, 31 Jul 2016, M. Nobis & A. Nobis s.n. (KRA 487177, 487178).
Taxonomic notes
The genus Achnatherum in Kyrgyzstan is represented by four species: A. caragana (Trin.) Nevski, A. jacquemontii (syn. A. botschantzevii Tzvel.), A. turcomanicum (Roshev.) Tzvel. and A. saposhnikovii (Roshev.) Nevski (syn. Timouria saposhnikovii Roshev.). In the Checklist flora of Kyrgyzstan (Lazkov & Sultanova, 2014) is also cited A. longiaristatum (Boiss. et Hausskn.) Roshev. (syn. Piptatherum longearistatum Boiss. et Hausskn., Stipa kurdystanica Bor), however, this species is endemic for Zagros Mts in Iran and Iraq (Freitag, 1985) and is not a component of Kyrgyz flora. During the field trip in central Tian Shan in 2016, the next species of the genus, A. sibiricum was found. The species is somewhat similar to young individuals of Neotrinia splendens (Trin.) M. Nobis, P.D. Gudkova & A. Nowak, however, well differs by having longer flowers (7–9 vs. 4–6.5 mm long), longer callus (0.5–0.7 vs. 0.2–0.4 mm long) and maize-like pattern of the lemma epidermis, typical for achnatheroid grasses (Nobis et al., 2019a, 2020; Romaschenko et al., 2012).
Key to the Achnatherum species in Kyrgyzstan
1. Awns bigeniculate, sometimes the lower bent weekly visible ........................................................ 2
– Awns straight, flexuous or unigeniculate ........................................................ 3
2. Awn slender, scabrous, 20–35 mm long, floret 4.5–6 mm long, callus 0.3–0.4 mm long, palea 1.5–2 mm shorter than lemma, glumes 5.5–7 mm long, glabrous ........................................................ A. jacquemontii
– Awn robust, scabrous, 14–20 mm long, floret 6–8 mm long, callus 0.5–0.7 mm long, palea 1–1.5 mm shorter than lemma, glumes 8–10 mm long, shortly pilose only along veins ........................................................ A. sibiricum
3. Panicle 2.5–5 cm long, compressed, spike-like, glumes 4–5.5 mm long, floret 2.5–3.5 mm long, awns 2.5–4 mm long ........................................................ A. saposhnikovii
– Panicle 10–40 cm long, compressed or open, glumes 4–8 mm long, floret 2.5–4 mm long, awns 4–15 mm long ........................................................ 4
4. Extravaginal shoots present, panicle compressed, awns persistent 10–15 mm long ........................................................ A. turcomanicum
– Extravaginal shoots absent, panicle open, awns caducosus 4–13 mm long ........................................................ A. caragana
Distribution and habitat
Achnatherum sibiricum is a widely distributed species. Its occurrence is known from the Caucasus throughout northern Central Asia to eastern Siberia (Tzvelev, 1976; Wu & Phillips, 2006). In Central Asia, it is known from Uzbekistan, Kazakhstan, and China. The new locality described here is the southernmost in the region, and the species is native to the Kyrgyz flora. The locality was found in 2015 and confirmed in 2016 (Figure S1 in the Supplementary material) and 2022. Probably, subsequent localities of the species can be found in the region. The population of the species found near the Issyk-Kul Lake consists of several tufts of A. sibiricum growing at the bottom of the rocky face of the mountain. The species occurs here together with other representatives of feather grasses, i.e., Neotrinia splendens, Stipa caucasica subsp. nikolai M. Nobis, A. Nobis & A. Nowak, and S. orientalis Trin.
Arrhenatherum elatius (L.) P. Beauv. ex J. Presl & C. Presl (Poaceae)
Synonyms: Arrhenatherum americanum P. Beauv., Arrhenatherum avenaceum (Scop.) P. Beauv., Arrhenatherum tuberosum F.W. Schultz, Avena avenaceum (Scop.) P. Beauv., Avena elata Salisb., Avena elata Forssk., Avena elatior L., Avena tuberosa Gilib., Avenastrum elatius (L.) Jess., Holcus avenaceus Scop., Hordeum avenaceum Wigg. ex P. Beauv.
Contributors: Marcin Nobis, Marta Krzempek
New records
TAJIKISTAN: Takob, Gissar Range, “Hissar-Darwaz Region A”, scrub in the valley of a stream - the right tributary of the Varzob River - near the Upper Varzob botanical station in Kondara - about 40 km to the N of Dushanbe, N 38°48′48″ / E 68°48′20″, elev. 1300 m, 13 Jun 2008, M. Nobis, M. Kozak, A. Nowak s.n. (KRA 434235, 435299, 435298); Dushanbe, Karamov Street, “South Tajikistan Region A”, Botanical Garden - near the paths, N 38°36′25″ / E 68°46′47″, elev. 880 m, 1 Jun 2007, M. Nobis s.n. (KRA 435779).
Taxonomic notes
Arrhenatherumelatius is the only representative of the genus Arrhenatherum in Central Asia (Romero-Zarco, 2011; Tzvelev & Probatova, 2019). The species is tetraploid and its basic chromosome number is x = 7; 2n = 4x = 28 (Pfitzenmeyer, 1962; Tzvelev & Probatova, 2019). It is tussock-forming, perennial grass with a long (up to 180 cm), erect, and subcylindrical stem. The plant possesses up to 20 cm long panicles, each with 50 to 100 spikelets.
Arrhenatherum elatius may be misidentified with A. bulbosum (Willd.) C. Presl, which grows in Europe, south-west Asia, and northern Africa (Tzvelev & Probatova, 2019) and is characterized by the occurrence of corms (organs of vegetative reproduction) at the base of the stems (Cussans et al., 1993).
Distribution and habitat
Arrhenatherum elatius is a species native to Europe and western Asia (Tzvelev, 1976). Its natural range of distribution reaches 70°N on the Atlantic coast of Norway (Pfitzenmeyer, 1962), its northern limit of occurrence is defined by the −6.5 °C January isotherm, and the eastern limit of its range is the Caucasus (Michalski et al., 2017; Pfitzenmeyer, 1962). Beyond Europe, it occurs in North Africa. It has also been introduced to North America, New Zealand, and Australia (Michalski et al., 2017; Pfitzenmeyer, 1962). The highest altitude at which A. elatius has been recorded is 1920 m a.s.l. in Europe, 3000 m a.s.l. in the Caucasus, and 1400–1600 m a.s.l. in Africa (Pfitzenmeyer, 1962).
Arrhenatherum elatius has never been recorded in the mountains of Central Asia in the wild. However, it has been cultivated in experimental fields within this area (Lazkov & Sennikov, 2014; Sidorenko, 1957). Recently, A. elatius has been recorded in two locations in Kyrgyzstan: the village of Kök-Say and the northern side of Issyk-Kul Lake. In Kök-Say village, the species is regarded as locally established and not threatening the native vegetation (Lazkov & Sennikov, 2014). In Tajikistan, A. elatius has been recorded in the scrub within the noname stream valley near the botanical station in Kondara (Figure S2 in the Supplementary material) as well as along paths in the botanical garden in Dushanbe. In Kondara, the occurrence of the species was confirmed in 2019, and it was classified as an established anthropophyte but not as invasive.
Asyneuma thomsonii (Hook.f.) Bornm. (Campanulaceae)
Synonyms: Campanula thomsonii Hook.f.
Contributors: Marcin Nobis, Ewelina Klichowska, Anna Wróbel, Arkadiusz Nowak
New record
KYRGYZSTAN: W Tian-Shan, Talas Ala-Too Range, Talas region, ca. 70 km to the SE of Talas city, near the road M41; shrubs, N 42°03′34″ / E 72°49′07″, elev. 1535 m, 28 Jun 2017, M. Nobis, E. Klichowska, A. Wróbel, A. Nowak (KRA 474789, 474787, 476524, 477004).
Taxonomic notes
The genus Asyneuma is represented in the flora of Kyrgyzstan by two species: A. argutum (Regel) Bornm. (syn.: A. baldshuanicum (B.Fedtsch.) Fed.; A. ramosum Pavl.; ?A. debile Fed.) and A. attenuatum (Franch.) Bornm. (syn. A. trautvetteri (B.Fedtsch.) Bornm.; A. strictum Wendelbo). During field studies in 2017, we found the third new species in the flora of Kyrgyzstan. In the result of comparing the morphological features included in different identification keys (e.g., Damboldt, 1970; Khassanov & Kodirov, 2017; Li, 1987) and revision of the herbarium materials of selected species belonging to the genus Asyneuma in LE, AA, TAD, KRA, K, E, we identified the newly found species as A. thomsonii. The specimens of the taxon well differ from all the other representatives of the genus in Kyrgyzstan by having glabrous stems, inflorescence branched, flowers loosely distributed (not in cymes distantly separated from each other), lower and middle stem leaves petiolate. Asyneuma thomsonii may be misidentified with morphologically similar A. japonicum that grows in eastern Asia and differs by having longer stems, longer calyx lobes (6–10 mm long), equal to or somewhat shorter than corolla, and ovate seeds, 0.9–1 mm long × 0.5–0.6 mm wide (Damboldt, 1970; Kozhevnikov, 1996). Asyneuma thomsonii is also well differentiated from the three above-mentioned species by molecular data. Based on SNPs derived from DArTseq as well as combined ITS and cpDNA analysis (Supplementary file; Figure 2), the specimens form a well-separated clade. During the revision of the herbarium materials of Asyneuma from Kyrgyzstan, we also found several specimens with a set of characters not matching the description neither of A. argutum nor A. thomsonii. The specimens have lower and middle stem leaves lanceolate to ovate and shortly petiolate (1–2 cm long) and inflorescence with flower organized in cymes distantly separated from each other [specimens examined: Ferganskkaya obl. Andizhanskk. u. Aralanbab, 30 May 1899, D. Litvinov (LE, 3 sheets); Dolina r. Aflatun (Namanganskago) pri vpadeni r. Yyalma-chaty, 25 Aug 1902, B. Fedtschenko (LE 2 sheets); U ozera Sary-Chelek, 22 Jul 1915, R.I. Roshevitz (LE)]. It is not excluded that these specimens might be a result of hybridization between A. thomsonii and A. argutum. However, due to the age of the plant material (the specimens were collected over one hundred years ago), we did not include them in the molecular analyses. The revision of this problematic and morphologically highly variable species group, using tools of integrative taxonomy, is badly needed. It is also worth mentioning that the phylogenetic position of Asyneuma as a separate genus is rather doubtful. It is generally clustered within the Campanula clade, within the subclade comprising species of Phyteuma, Sergia, Cylindrocarpa, and several species of bellflowers (Jones et al., 2017; Xu & Hong, 2021). Xu and Hong (2021) proposed to merge all of the species of this mentioned above subclade within the common genus Phyteuma with the new appropriate circumscription. Nevertheless, taking into account that the phylogenetic relationships between Campanula and other genera within Campanulaceae are pretty puzzling, it is worth considering a monophyletic approach to the genus and reinclusion of species from such genera as, e.g., Asyneuma, Phyteuma, Sergia, Cylindrocarpa, Petromerula or Adenophora to Campanula, just as it was already proposed by Tojibaev et al. (2021) and some other previous authors. Such a broader monophyletic circumscription of Campanula (including a number of subgenera and sections) will reflect the close evolutionary relationships in the group and serve the goal of nomenclatural stability within the genus.
Figure 2
Phylogenetic relationship estimated from (A) Neighbor-Joining phylogenetic tree based on Euclidean distance (DArT, 459 SNPs) and (B) Bayesian inference of phylogeny (concatenated ITS and cpDNA, 2128 bp). For methods, see Supplementary file.
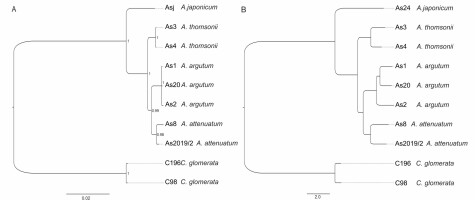
Distribution and habitat
Asyneuma thomsonii is a central Asian-mountain species distributed in high mountains of the western Himalayas in India (Kashmir), the Karakorum Mts in Pakistan and Afghanistan, the western Pamir-Alai Mts in Tajikistan and Uzbekistan, as well as the Tian Shan Mts in Kyrgyzstan (Damboldt, 1970; Khassanov & Kodirov, 2017). During field research in 2017 and 2022, the species was found in the Talasski Ala-Too on roadsides and within light shrubs and forests (Figure S3 in the Supplementary material).
Calamagrostis emodensis Griseb. (Poaceae)
Contributors: Beata Paszko, Wen-Li Chen
New records
CHINA. Gansu: Baishuijiang Nature Reserve, Qiujiaba, edge of a forest, elev. 2400–2700 m, [32.899°N, 104.591°E], 5 Sept 2007, Baishuijiang Team 5660 (PE!); Liujiaping, Qixingoudonghe, under forest, elev. 1756 m, [33.008°N, 104.744°E], 21 Aug 2006, Baishuijiang Team 2470 (PE!).
Taxonomic notes
Calamagrostis emodensis is a member of the genus Calamagrostis Adans. sect. Calamagrostis Dumort. which comprises a few closely related groups of taxonomically difficult species (Paszko & Ma, 2011; Tzvelev & Probatova, 2019). In the recent treatment of Calamagrostis for China, Lu and Phillips (2006) have defined it very narrowly and recognized six species: C. emodensis Griseb., C. pseudophragmites (Haller f.) Koeler, C. hedinii Pilg., C. macrolepis Litv., C. epigejos (L.) Roth and C. kengii T. F. Wang. Paszko and Ma (2011) revealed that most Chinese collections of C. epigejos were incorrectly identified, and most represent C. extremiorientalis (Tzvel.) Prob., which is also the older name for C. kengii. Calamagrostis emodensis (Figure 3) is regarded as a very distinctive species in the section Calamagrostis, and it is quite easy to distinguish from the members of the C. pseudophragmites complex. It is usually characterized by having a five-veined lemma with a deeply two-toothed apex, awn 5–9 mm arising between teeth, relatively broad leaf blades, a nodding panicle (Figure 3), and its florets containing one stamen with a single plump anther (Bor, 1960; Lu & Phillips, 2006). Calamagrostis emodensis differs from C. pseudophragmites complex by relative awn insertion on the lemma (exerted between deep teeth above the midpoint versus apical or subapical), longer awns (5.0–8.5 mm versus 1–4 mm), shorter leaf ligules (0.5–4.0 mm versus 2–26 mm) and shorter anthers (0.6–1.1 mm versus 1.0–2.3 mm) (Paszko, 2012). Recently, Paszko (2013) determined the correct taxonomic placement of Calamagrostis emodensis var. breviseta Hack., which was incorrectly linked with C. emodensis at the time of description. This variety is conspecific with C. macrolepis Litv.
Figure 3
Calamagrostis emodensis Griseb. (A) Panicle of C. emodensis, Mt. Cangshan, Yunnan (China), 24 August 2010. Photo. B. Paszko. (B) Distribution map for C. emodensis produced using SimpleMappr (http://www.simplemappr.net/). Two new localities in Gansu Province (China) are marked by red triangles. For a list of localities, see Supplementary file.
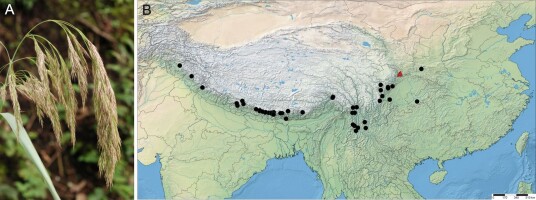
Distribution and habitat
Calamagrostis emodensis extends from the Himalaya Shan through Southwest China (Sichuan, SE Xizang, Yunnan) to central China, Shaanxi, and southern Gansu. It was known hitherto from Nepal, Bhutan, China (Shaanxi, Sichuan, Xizang, Yunnan), India (Himachal Pradesh (disputed area), Jammu & Kashmir (disputed area), Sikkim, Uttarakhand), and Pakistan (Hazara) (Bor, 1960; Cope, 1982; Lu & Phillips, 2006; Noltie, 2000; Press et al., 2000). The first locality of C. emodensis has recently been recorded from Kachin State in Northeast Myanmar (Burma) by Paszko (in Nobis et al., 2014). Specimens of C. garhwalensis can be misidentified with C. emodensis. Here we provide the first two records of Calamagrostis emodensis from Wenxian County in southern Gansu Province, North-Central China, where Calamagrostis emodensis was collected in the Baishuijiang National Nature Reserve. These localities are located at the northern limit of its geographical range (Figure 3). Calamagrostis emodensis is native to the woodlands of Himalayas, southwestern China, and Central China. It is common on landslides in the fir zone, wet cliffs, and stream sides in the mixed broadleaved-pine forests, banks, and gravels by rivers. It grows at an elevation between 1750 and 3660 (−4100) m.
Calamagrostis obtusata Trin. (Poaceae)
Synonyms: Calamagrostis agrostioides Matuszk.
Contributors: Beata Paszko, Bing Liu
New records
CHINA. Nei Mongol: Hexigten Banner, Huanggangliang Forest Farm, Dadonggou, Da’etou, under [Betula platyphylla] forest, elev. 1720 m, 43.521°N, 117.339°E, 28 Jul 2008, Chifeng Exped. Team (Y.B. Sun, B. Liu et al. 2-092) (PE02012002). Shaanxi: [Ningwu Co., Dongzhai Township, Ta keou (between villages Bagouwan and Donglougou), en forêt, 38.862°N, 112.069°E], 9 Jul 1922, E. Licent 6739 (K, PE00449999, W).
Taxonomic notes
Calamagrostis obtusata is a member of the genus Calamagrostis Adans. sect. Deyeuxia (Clarion ex P. Beauv.) Dumort., which comprises several closely related groups of taxonomically difficult species, including the species group of C. obtusata. This group comprises four close relatives: C. obtusata Trin., C. chalybaea (Laest.) Fr., C. pavlovii Roshev. and C. krylovii Reverd. (Tzvelev & Probatova, 2019). In the Flora of China, this group of taxa should be treated in the genus Deyeuxia Clarion ex P. Beauv. (Lu & Phillips, 2006). Till now, none of the species from the C. obtusata aggregate has been reported from China in the genus Deyeuxia (Lu et al., 2006) or Calamagrostis (Lu & Phillips, 2006). Recently, several new nomenclatural and taxonomic novelties have been discovered in the genus Deyeuxia in China (e.g., Liu & Paszko, 2020; Paszko et al., 2016a, 2016b; Paszko & Soreng, 2013).
Chinese specimens of Calamagrostis obtusata were misidentified with C. stricta (Timm) Koeler or C. purpurea (Trin.) Trin. Calamagrostis obtusata differs from C. stricta by a lower ratio of callus hairs to lemma length (0.25–0.76 vs. 0.8–1.2), higher ratio palea to lemma length (0.85–1.05 vs. 0.7–0.85), hairy leaf collar (vs. glabrous in C. stricta), flat and glabrous upper leaf surface (vs. conspicuously scabrous and covers with high ribs and deep furrows in C. stricta) and lower ratio callus hairs to lemma length (0.25–0.76 vs. 0.7–1.2). Calamagrostis obtusata differs from C. purpurea by the lower ratio of callus hairs to lemma length (0.25–0.76 vs. 0.8–1.2), hairy leaf collar (vs. glabrous in C. purpurea), generative culm branching absent (vs. usually present in C. purpurea).
Distribution and habitat
Calamagrostis obtusta was described by Trinius (1824) based on a specimen collected in 1818, probably by Ernest Gottfried Haupt (1795–1862), in the vicinity of Tobolsk, a Russian city located in central Siberia. Calamagrostis obtusata is an East-European-Siberian species that has a wide distribution in Russia. It occurs from East European Russia through Ural Mts and across Eastern and Western Siberia to Russian Far Eastern regions. Its most southern localities were recorded in Kazakhstan and Mongolia (Gamayunova, 1956; Grubov, 1982; Ivanova, 1990; Tzvelev, 1976). In Kazakhstan, C. obtusata grows in its eastern part in the mountains of Altai, Dzungarian Alatau, and Tarbagatay (Gamayunova, 1956), which lie on China–Kazakhstan border. Whereas in Mongolia, it is recorded in its central part, in the Khangai and Khentii Mts (Grubov, 1982).
Here, Calamagrostis obtusata is reported as new to China. The first national records are provided from two Chinese provinces, Nei Mongol and Shaanxi (Figure S4 in the Supplementary material). The first more recent collection was made on 28 July 2008 by the Chifeng Expedition Team in the vicinity of Hexigten Qi (Hexigten Banner, Chifeng City, Nei Mongol) in the mixed forest of Larix principis-rupprechtii Mayr and Betula platyphylla Suk. (Figure 4).
Figure 4
Calamagrostis obtusata Trin. and its habitat at the locality in Nei Mongol (China): Hexigten Banner, Huanggangliang Forest Farm, Dadonggou, Da’etou, 11 September 2008 (A) Culm of C. obtusata; (B) panicle of C. obtusata; (C) Betula platyphylla forest. Photo. Bing Liu (A–C).

The record from Shaanxi is based on a collection made by Emile Licent (1876–1952) on 9 July 1922 in the forest near Ta keou (between villages Bagouwan and Donglougou, Ningwu Co., Shaanxi), in the area of Luya Shan (Licent, 1924) (Licent 6739; K, PE, W) (Figure S4 in the Supplementary material). Licent’s collections of vascular plants from China, made between 1914 and 1933, are spread around different herbaria, such as K, P, PE, W, and maybe others too. In most cases, collection labels provide only information about the collection date, collector name, and collection number (especially those housed at the PE herbarium), therefore they can be neglected by scientists. Sometimes information about collection locality provided on the label can be misleading, like in the case of the Kew sheet bearing Calamagrostis obtusata. To find or compare a historical locality of Emile Licent with up-to-date state, we used information from Licent’s collection’s accounts (Licent, 1924, 1936), which cover two decades of his scientific travels. Licent aimed to gather as much material and information as possible, cataloging his travels in two huge volumes. These volumes include travel itineraries, travel journals, maps, and reproductions of photographs taken along the routes, the exact locations of which are indicated on the maps (Manias, 2017; Swanton, 1927). The area of Luya Shan, located in the northeastern corner of the Luliang Mts, provides suitable habitats for C. obtusata. Here, conifer forests comprised Picea spp. and Larix spp. grow with a relatively high cover of 31% (Carpenter, 2018).
Dittrichia graveolens (L.) Greuter (Asteraceae)
Synonyms: Alunia graveolens (L.) Lindl., Erigeron graveolens L., Helenium graveolens (L.) Kuntze, Inula graveolens (L.) Desf.
Contributors: Arkadiusz Nowak, Sebastian Świerszcz
New record
TAJIKISTAN: Varzob River Valley in Dushanbe, N 38.49511, E 68.76975, elev. 720–750 m, ruderal vegetation, 09 Oct 2021, A. Nowak, S. Świerszcz s.n. (OPUN – Herbarium of Opole University).
Taxonomic notes
The genus Dittrichia Greuter is represented by two species, i.e., Dittrichia viscosa (L.) Greuter and Dittrichia graveolens (L.) Greuter. Dittrichia graveolens, first described as Erigeron graveolens L., is an erect and densely glandular annual plant (20–70 cm tall). Additionally, D. graveolens can be distinguished from D. viscosa by shorter ligules (4–7 mm), not or scarcely exceeding the involucre. Among the representatives of the genus Dittrichia, only D. graveolens has a native occurrence in southwest Asia (Ball, 1976).
Distribution and habitat
Dittrichia graveolens, is a plant of Mediterranean distributional type (Ball, 1976; Brullo & de Marco, 2000). In Central Europe, the rapid expansion of this species has been reported in road verges, particularly motorways (Frajman & Kaligarič, 2009; Király et al., 2014; Kocián, 2015). It has also been introduced in North America, Australia, and South Africa, where it causes significant threats to crops (e.g., Brownsey et al., 2013; Kloot, 1987).
During field research in the southern districts of Dushanbe in October 2021, the species was found in road verges of an industrial zone and transportation base in the city outskirts and the sediment ponds of the city’s sewage treatment plant (Figure 5). The population is huge, comprising tens of thousands of individuals that form dense vegetation in ruderal habitats. Numerous populations have not been observed on the banks of the Varzob River, but its proximity poses a severe threat to the spread of this invasive species. There is a high probability that seeds of Dittrichia graveolens will spread rapidly along the Varzob River, the Kafirnighan River (which flows into it not far from the found location), and further south also along the other river valleys. It is worth noting that floristic surveys conducted in the area of the sewage treatment plant between 2006 and 2010 did not confirm the presence of this species. Dittrichia graveolens has rapidly vast overgrown lands of man-made habitats recently. The size of the population proves that the species is an established component in the flora of Tajikistan.
In Dushanbe, the population of D. graveolens was accompanied by: Abutilon theophrasti, Alhagi pseudalhagi subsp. kirghisorum, Amaranthus retroflexus, Bromus scoparius, B. tectorum, Chenopodium strictum, Crithopsis delileana, Cynodon dactylon, Eleusine indica, Heliotropium ellipticum, Lactuca serriola, Medicago orthoceras, Parapholis incurva, Polygonum paronychioides, Silybum marianum, Sisymbrium loeselii, Verbascum erianthum and Vulpia myuros (nomenclature of species after POWO, 2022).
Draba fladnizensis Wulfen (Brassicaceae)
Contributors: †Paweł Kauzal, Antoni Zięba, Sławomir Wróbel
New record
POLAND: Western Carpathians – Tatra Mts – High Tatras – Żabi Szczyt Niżni – crevices and rock ledges of the granite wall, exposure: N, elev. ca. 2030 m, EG6029 (ATPOL grid), 21 Jun 2022, S. Wróbel s.n. (KRA).
Taxonomic notes
Draba fladnizensis is a tiny perennial white-flower plant forming tufts with a various number of leaf rosettes. Together with a yellow-flower Draba aizoides L. and three other white-flower species – Draba dubia Suter, Draba siliquosa M.Bieb., and Draba tomentosa Clairv. (Delimat et al., 2014; Pawłowski, 1956; Ronikier, 2014; Wróbel et al., 2014), D. fladnizensis is the fifth species of Draba currently noted in the Polish Tatra Mts. Draba fladnizensis could be distinguished from other white-flower Draba species occurring in the Tatras by characteristic leaves – lacking stellate trichomes, hairless on a surface, and only with straight trichomes on the margins (Figure 6), as well as by a stem – glabrous and typically leafless (Peniašteková & Kliment, 2002).
Figure 6
Draba fladnizensis on Żabi Szczyt Niżni in the Tatra Mts, Poland: (A) a tuft of leaf rosettes with blooming shoots; (B) leaf rosettes – hairless on a surface and only with straight trichomes on the margins; (C) a habitat of crevices and narrow rock ledges located at the lower part of a vertical granite rock wall; (D) rock crevices colonized by chasmophytes including tufts of D. fladnizensis (photo. S. Wróbel, 2022).

Distribution and habitat
Draba fladnizensis is a circumpolar plant occurring in the arctic zone of Eurasia and North America. It also grows in higher mountain ranges of the Northern Hemisphere. In Europe, except the polar zone, Draba fladnizensis grows in the Scandinavian Mts, the Pyrenees, the Alps, and the Carpathians (Meusel et al., 1965). In the Carpathians, it was detected in a few localities in Romania: the Bucegi Mts – the Southern Carpathians, the Rodna Mts – the Eastern Carpathians (Sârbu et al., 2013), and in Slovakia: the Belianske Tatras – the Western Carpathians (Peniašteková & Kliment, 2002). Draba fladnizensis grows in rock crevices, ledges, and screes. This plant occurs predominantly on quartzitic rocks, often dewed with water rich in calcium carbonate, which leaks from higher rocks (Chrtek et al., 1999).
Draba fladnizensis was found in the Polish Tatras by Paweł Kauzal on the 4th of July 2018. It is the first record of this species in Poland. The population described in detail on the 21st of June 2022, consists of 23 tufts, with a few to dozen rosettes, which formed 48 generative shoots in total (Figure 6). The plants are scattered on an area of ca. 50 m2 in crevices and narrow rock ledges, located at the lower part of a vertical granite rock wall. In the vicinity of Draba fladnizensis, we detected the following species of vascular plants: Carex fuliginosa, Festuca airoides, Lloydia serotina, Oxyria digyna, Pedicularis verticillata, Ranunculus alpestris, R. glacialis, Saxifraga androsacea, S. oppositifolia, S. wahlenbergii, Silene acaulis (nomenclature of the species is given after Mirek et al., 2020).
Due to the very small number of mature individuals (<50) of the discovered population and its limited spatial coverage, Draba fladnizensis should be considered a critically endangered species (CR) in Poland, according to the D criterion of IUCN Red List Categories (IUCN, 2012).
Gentiana orbicularis Schur (Gentianaceae)
Synonyms: Gentiana favratii Rittener, Gentiana brachyphylla Vill. subsp. favratii (Rittener) Tutin
Contributors: Sławomir Wróbel, Anna Wróbel
New records
POLAND: Western Carpathian Mts, Tatra Mts, Liliowe Turnie (Baniste), elev. 1940 m, exp. NW, alpine grasslands, DG6921 (ATPOL grid), 14 Jun 2022, S. Wróbel s.n. (KRA); Western Carpathian Mts, Tatra Mts, Starorobociański Wierch, elev. 1970 m, exp. N, alpine grasslands, DG6819 (ATPOL grid), 20 Jun 2022, S. Wróbel s.n. (KRA).
Taxonomic notes
According to the latest integrative revision (Hämmerli, 2007), G. orbicularis is considered a separate species within Gentiana Sect. Calathianae Froel. Moreover, it is well-distinguished by its morphology and DNA profile. The species is most similar to Gentiana verna L., which is a much more widespread taxon occurring throughout South and Central Europe, on the British Isles, in the Caucasus, arctic Russia and reaching Siberia and Mongolia in the east (Tutin, 1972). In the Carpathians, G. verna is most frequently associated with limestone communities (Bertová, 1984; Jasiewicz, 1971).
Both G. orbicularis and G. verna produce basal leaf rosette; however, they differ in the leaf shape (Hämmerli, 2007; Jasiewicz, 1971; Tutin, 1972). Gentiana orbicularis has broadly elliptic, suborbicular, ovate, or obovate rosette leaves that are ca. 1 cm long with more or less rounded apex. On the contrary, G. verna has lanceolate to elliptic rosette leaves that are up to 3 cm long with an acute or obtuse apex (Figure 7). Moreover, the longest rosette leaves of G. orbicularis are no or little longer than its cauline leaves. On the other hand, the longest rosette leaves of G. verna are about twice as long as its cauline leaves. Some resemblance to G. orbicularis could also be attributed to Gentiana brachyphylla Vill. s.str., a species largely restricted to the Alps, which is well-distinguished by its DNA profile (Hämmerli, 2007).
Figure 7
Gentiana orbicularis in the Western Tatra Mts, Poland: (A) a cluster of vegetative and blooming shoots on Liliowe Turnie; (B) habitat – alpine grasslands on Starorobociański Wierch (photo. S. Wróbel, 2017 and 2022); (C) morphological comparison of blooming shoots between Gentiana orbicularis and Gentiana verna, and (D) Maximum-Likelihood tree inferred from ITS1–5.8 S–ITS2 region of nuclear ribosomal DNA (numbers near tree nodes indicate bootstrap support values from 1000 replicates; scale bar indicates the proportion of differences under a General Time Reversible model; the tree was rooted by a sequence of Gentiana asclepiadea; abbreviations: PL – Poland, CH – Switzerland, BG – Bulgaria).
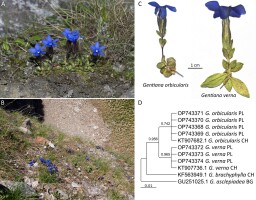
Due to the phenotypic plasticity observed in Gentiana and the novelty of our discovery, we combined morphological identification with DNA barcoding (Figure 7) using ITS1-5.8S-ITS2 marker of nuclear ribosomal DNA (Supplementary file). Our integrative approach clearly supports the identification of the individuals from the Tatra Mts as G. orbicularis.
Distribution and habitat
Gentiana orbicularis is an alpine species occurring throughout the Alps and in a few isolated localities in the Carpathians (Hämmerli, 2007). In the latter, G. orbicularis was observed in the southern part of the range, in the Bucegi Mts, Romania, in sunny places on rocks and skeletal soils in the alpine zone (Beldie, 1967). The species was also reported from one locality in the Western Carpathians, in the Slovakian Belianske Tatra Mts on Predné Jatky Mt. at ca. 2000 m a.s.l. (Bertová, 1984; Fröhner, 1968). However, the origin and current status of this population remain unclear – according to Dostál, G. orbicularis presumably appeared in this locality after human-caused introduction (Dostál, 1989).
During the field research on 4 July 2017 in the Western Tatra Mts the species was found in two localities within Tatra National Park, Poland, Liliowe Turnie between 1930–1950 m a.s.l. on a slope with north-western exposure, and Starorobociański Wierch between 1960–2010 m a.s.l. on a slope with northern exposure. Both localities are situated in the alpine zone on steep slopes where strongly eroded vertical or overhanging rocks occur. Gentiana orbicularis grew in the Festuco versicoloris-Agrostietum alpinae Pawł., Sokoł. et Wallisch 1928 community established on the mylonite metamorphic rock. In total, the species formed ca. 100 clusters and several hundred blooming shoots. The population sizes were similar, and G. orbicularis was scattered throughout the area of ca. 400 m2 on Liliowe Turnie and ca. 1000 m2 on Starorobociański Wierch. In both localities, G. orbicularis was accompanied by: Alchemilla sp., Antennaria carpatica, Anthoxanthum alpinum, Campanula alpina, Cardaminopsis neglecta, Carex sempervirens, Cerastium tatrae, Doronicum clusii, Festuca airoides, F. versicolor, Geum reptans, Huperzia selago, Lloydia serotina, Luzula alpino-pilosa, Minuartia verna, Mutellina purpurea, Oreochloa disticha, Oxyria digyna, Pedicularis oederi, Poa alpina, Primula minima, Ranunculus alpestris, Salix reticulata, Saxifraga androsacea, S. moschata, S. oppositifolia, S. paniculata, Sesleria tatrae, Silene acaulis, Soldanella carpatica, Swertia perennis, Veronica aphylla, and Viola biflora (nomenclature of species is given after Mirek et al., 2020).
Due to very restricted distribution and a small number of mature individuals (<250), G. orbicularis should be classified as endangered (EN) in Poland according to IUCN 2012 – EN, criterion D (IUCN, 2012). Both populations were monitored in June 2022 and showed no signs of a decline since the first observation in 2017.
Geranium pyrenaicum Burm.f. (Geraniaceae)
Synonyms: Geranium rotundifolium var. grandiflorum Wahlenb.
Contributors: Arkadiusz Nowak, Sebastian Świerszcz
New record
TAJIKISTAN: Dushanbe, N 38.55641, E 68.79384, ruderal vegetation on tracks and around platforms of the central railway station, elev. 720–780 m, 10 Oct 2021, A. Nowak, S. Świerszcz s.n. (OPUN).
Taxonomic notes
The genus Geranium is represented by 12 species in Tajikistan (Ovchinnikov, 1981). Geranium pyrenaicum is a perennial plant with stems 25–70 cm tall. It can be distinguished from Geranium sibiricum, which has lanceolate, acute leaf-lobes, and patent hairs on the mericarp, whereas G. pyrenaicum has cuneate, truncate leaf-lobes, and adpressed hairs on the mericarp (Webb & Ferguson, 1968).
Distribution and habitat
The native range of Geranium pyrenaicum comprises the Mediterranean and sub-Mediterranean regions with Albania, Algeria, Austria, Bulgaria, Corse, Czech Republic, France, Germany, Great Britain, Greece, Hungary, Iran, Ireland, Italy, Crimea, Lebanon-Syria, Morocco, North Caucasus, Portugal, Romania, Sicilia, Spain, Switzerland, Transcaucasia, Tunisia, European part of Turkey, Ukraine, and Yugoslavia (GBIF, 2022; POWO, 2022). The species is also known as being introduced in the Baltic States, Belarus, Belgium, central European part of Russia, Denmark, Finland, the Netherlands, Norway, Poland, Sweden, and West Siberia. It was also noticed in North America (California, Michigan, New York, Vermont, Ontario, Québec), South America (Chile), and Australia (GBIF, 2022; POWO, 2022; Meusel et al., 1978; Seebens et al., 2017).
During the field research on the 10th of October 2021, the species was found along railway tracks and ruderal habitats of storage yards and railway sidings of Dushanbe Main Railway Station (Figure 8). The population size was huge with an uncountable number of individuals. The population occupies bedded areas of railway embankments, roadside verges, and the vicinity of railway platforms. The population certainly comes from materials carried with rail transport. Dushanbe is the central station in Tajikistan, hence the species can be expected to spread rapidly both to southern areas (Kulob, Shartuz) and to west neighboring Uzbekistan.
Figure 8
Geranium pyrenaicum in ruderal vegetation near railway tracks in Dushanbe (photo. A. Nowak, 2021).

In Dushanbe, the population of G. pyrenaicum was accompanied by: Abutilon theophrasti, Alhagi pseudalhagi subsp. kirghisorum, Alyssum dasycarpum, Arabidopsis thaliana var. pusilla, Bromus tectorum, Cardamine hirsuta, Cynodon dactylon, Erophila verna, Crithopsis delileana, Hordeum murinum subsp. leporinum, Lactuca serriola, Microcephala lamellata, Oxalis corniculata, Phleum graecum, Potentilla indica, Sisymbrium loeselii, Tribulus terrestris and Vulpia myuros (nomenclature of species after POWO, 2022).
Helianthus giganteus L. (Asteraceae)
Contributors: Marcin Nobis, Agnieszka Nobis
New record
POLAND: Vistula River valley, Połaniec, roadside near the ferry crossing, elev. 162 m, EF29 (ATPOL grid), 26 Sept 2016, M. Nobis s.n. (KRA).
Taxonomic notes
The genus Helianthus is represented in the flora of Poland by six introduced species (Mirek et al., 2020). Three of them, namely H. decapetalus L., H. × laetiflorus Pers., and H. tuberosus L. are established anthropophytes, whereas the remaining species, namely H. annuus L., H. salicifolisus A. Dietr., and H. serotinus Dietr., are cultivated and sporadically escaping from cultivation therefore classified in Poland as ephemerophytes. During the field studies in the Vistula River valley in 2016, we found the next taxon, i.e., H. giganteus. The species is well distinguished from all the other Helianthus species in Poland by having lanceolate leaves on the stem and involucral bracts adpressed, narrow (1.2–2 mm wide) and acute at the apex (Figure 9; Jäger et al., 2016; Rutkowski, 2017; Schilling, 2006).
Key to the perennial, locally established species of sunflowers occurring in Poland
1. Stems glabrous ........................................................ 2
– Stems hairy thought (or at least in the upper half) ........................................................ 3
2. Leaves linear to lanceolate, 0.2–1.2 cm wide, cuneate, (sub-) entire at margin, phyllaries 40–50, linear to lance-linear, 12–20 × 1.8–2 mm (not surpassing discs length), apices long-attenuate ........................................................ H. salicifolius
– Leaves lanceolate to ovate, 4–10 cm wide, bases rounded to cuneate, at margins serrate, phyllaries 20–25, lance-linear to lanceolate, 11–16 × 2–3 mm (sometimes leaflike, longest surpassing discs by 1/2+ their lengths) ........................................................ H. decapetalus
3. Involucral bracts usually markedly unequal (the outermost shorter than the inner), always closely appressed, leaves lanceolate to lance-ovate, 10–25 × 2–8 cm, bases cuneate ........................................................ H. × laetiflorus
– Involucral bracts subequal, loosely appressed or spreading, leaves lanceolate to ovate ........................................................ 4
4. Leaves lanceolate 1.5–3.5 cm wide, shortly petiolate, phyllaries 20–25 (loose or spreading), linear, 1.2–2 mm wide, (margins usually ciliate) apices acute ........................................................ H. giganteus
– Leaves wider, lanceolate to ovate, 4–15 cm wide, phyllaries 20–25, lanceolate, 2–4 mm wide, loosely appressed ........................................................ 5
5. Stem rough hairy throughout, phyllaries not surpassing disc diameter ........................................................ H. tuberosus
– Stem glabrous, at least in the lower half, phyllaries surpassing disc diameter ........................................................ H. decapetalus
Distribution and habitat
Helianthus giganteus is native to the eastern United States and eastern and central Canada (Schilling, 2006). In its natural range, it grows on wet meadows or swamps, often near river banks. The species was introduced to Europe as an ornamental plant, and sometimes it is observed as a locally established anthropophyte (epeco- or rarely agriophyte). In Połaniec, the species population comprised several dozen specimens and grew on the roadside, near the ferry crossing in the Vistula River valley (Figure 9). The status of the species in the flora of Poland requires further studies. However, it can be much more frequent since it can be misidentified with H. tuberosus or H. × laetiflorus.
Hieracium piliferum Hoppe (Asteraceae)
Contributors: Sławomir Wróbel, Anna Wróbel
New record
POLAND: Western Carpathian Mts, Tatra Mts, High Tatra Mts, Rybi Potok Valley, Miedziane, Szeroki Żleb, acidophilic alpine grassland on the granite rock, elev. ca. 1660 m, exp. SE, EG6017 (ATPOL grid), 27 Jun 2022, S. Wróbel s.n. (KRA).
Taxonomic notes
Hieracium piliferum is the only representative of the section Barbata Gremli in the Polish flora (Jasiewicz, 1980). In the Tatra Mts, it could primarily be confused with morphologically similar and much widespread Hieracium alpinum L., and possibly also with Hieracium villosum Jacq. Hieracium piliferum has eglandulose leaf margins, whereas H. alpinum has glandulose leaf margins. Hieracium piliferum forms leafless stems (or with one sessile leaf) ending in a single inflorescence (Figure 10), while H. villosum produces stems with numerous amplexicaul cauline leaves and several flower heads (rarely one). The identification key to these species is presented in the work of Szeląg (2001).
Figure 10
Hieracium piliferum on the Miedziane Mt., the High Tatra Mts, Poland: (A) habitat within acidophilic alpine grasslands in the Szeroki Żleb; (B) mature leaf rosettes with blooming shoots (photo. S. Wróbel, 2022).

Distribution and habitat
Hieracium piliferum is a European mountain species with a core distribution area in the Alps. The plant also occurs in isolated localities in other European ranges, including the Pyrenees, the Massif Central, the Dinaric Alps, and the Carpathians (Meusel & Jäger, 1992). In the Southern Carpathians, H. piliferum was recorded in the Cernei Mts based on the specimens collected by Rochel at the beginning of the XIX century (Szeląg, 2001). However, the status of this locality remained uncertain (Meusel & Jäger, 1992), and its existence requires verification in the field (Sârbu et al., 2013). In the Western Carpathians, H. piliferum was observed only in the High Tatra Mts, within alpine grasslands established on a silicate substrate – in one locality within the Slovakian part of the range in the Valley of Piat Spišských plies, where species was not found afterwards (Szeląg, 2001), and in two localities on the Polish side in the Rybi Potok Valley, first one on the northern slope of the Mięguszowiecki Szczyt Wielki (Pawłowski et al., 1929), while the second one above Morskie Oko lake (Szeląg, 2001). Despite the attempts to confirm the species occurrence in the Polish Tatra Mts during field research in 2011 and 2012, H. piliferum was observed in neither of the previously recorded localities and, therefore, was regarded as extinct (EX or RE) in Poland (Kaźmierczakowa et al., 2016; Szeląg & Delimat, 2014)
On 27 June 2022, H. piliferum was rediscovered in the High Tatra Mts, within the Tatra National Park, Poland. The species was found in a new locality in the Rybi Potok Valley, in the Szeroki Żleb on the Miedziane Mt. at ca. 1660 m a.s.l. on the slope with south-eastern exposure. The population consisted of 113 leaf rosettes in total, including 86 rosettes with mature blooming shoots (Figure 10). The individuals were scattered throughout the area of ca. 50 m2 within acidophilic alpine grasslands surrounded by the mountain pine thickets. The plant community included the species distinctive to the classes Juncetea trifidi Hadač in Klika et Hadač 1944 and Nardo-Callunetea Prsg 1949, with the admixture of some limestone-related plants. Hieracium piliferum was accompanied by: Agrostis rupestris, Antennaria dioica, Avenula versicolor, Calamagrostis villosa, Calluna vulgaris, Campanula polymorpha, Carex sempervirens, Festuca airoides, Gentiana asclepiadea, Gymnadenia conopsea, Hieracium alpinum, H. murorum, Hypochoeris uniflora, Leontodon hispidus, L. pseudotaraxaci, Nardus stricta, Pinus mugo, Pulsatilla alba, Rhinanthus alpinus, Salix silesiaca, Solidago alpestris, Thesium alpinum, Thymus alpestris, Vaccinium myrtillus, V. vitis-idaea, and Veratrum lobelianum (nomenclature of the species is given after Mirek et al., 2020).
Currently, H. piliferum in Poland should be treated as critically endangered (CR) according to criteria B2a and B2b (IUCN, 2012).
Orobanche bartlingii Griseb. (Orobanchaceae)
Contributors: Renata Piwowarczyk, Óscar Sánchez Pedraja
New record
TURKEY: prov. Batum, pr. Mamanat [formerly Mamanati, today Demirciler, Borçka district of Artvin Province in Turkey] ad cacumen m. Moghven, in pratis iter fruticetis, elev. 2900’, 15 Jun 1902, Alexeenko & Woronow (TGM53196, TGM53198) [as O. owerinii by Melikischvili].
Taxonomic notes
The flora of Turkey consists of ca. 39 species of Orobanche s.l. (including Phelipanche Pomel; Davis et al., 1988; Gilli, 1982; Zare & Dönmez, 2013). However, the distribution of this genus in Turkey is not sufficiently known. Orobanche alsatica is a polymorphic aggregate, and comprises parasites of Apiaceae species: Orobanche alsatica Kirschl., s. str., parasitize mainly Peucedanum sp., such as Peucedanum cervaria(L.) Lapeyr., P. alsaticum L., while O. bartlingii Griseb., parasitize mainly of Seseli sp., e.g., Seseli libanotis (L.) W.D.J. Koch (syn. Libanotis pyrenaica (L.) Bourg.) and S. transcaucasicum (Schischk.) Pimenov & Sdobnina (syn. L. transcaucasica Schischk.). Orobanche bartlingii is morphologically similar and closely related to O. alsatica. To the characters that let to distinguished the two taxa belong: corolla (20–25 mm long with regularly and strongly curved dorsal line vs. 12–17 mm long with evenly curved at the base, respectively), style glandular pubescent vs. glabrous or rarely glandular-pubescent, stamens inserted 4–7 mm vs. 1–3 mm above base of the corolla tube (Pujadas Salvà & Gómez García, 2000; Piwowarczyk et al., 2018, 2019). The differences are also clearly observed in seed and pollen micromorphology (Piwowarczyk et al., 2014, 2015), as well as in hosts (Peucedanum vs. Seseli, respectively). Moreover, molecular analysis of samples of these two species showed that they are in a close relationship, although, ITS tree indicates that these species are clearly separated (Piwowarczyk et al., 2018, 2021).
Distribution and habitat
Orobanche bartlingii is a Eurasian species, occurring from Spain (Pyrenees mountain range), through Central and Eastern Europe and Russia to Siberia, and to Kazakhstan, Kyrgyzstan, and Caucasus (Piwowarczyk et al., 2019; Sánchez Pedraja et al., 2016). During the revision of the herbarium TGM, a new locality of O. bartlingii has been found from NE Turkey (Anatolian Peninsula) in the almost Caucasian, Artvin Province, collected in the mountain meadow near the shrubs at ca. 900 m a.s.l. (Figure S5 in the Supplementary material). The species is a new, native taxon to the flora of this country.
Stipa × balkanabatica M. Nobis & P.D. Gudkova (Poaceae)
Contributors: Marcin Nobis, Ewelina Klichowska
New records:
TAJIKISTAN: East Tajikistanian (A subregion), Kyzylsu River Valley, S of Alga settl., ca. 14 km NE of Damburacha, steppe on the hill, 39°19′43.48″N / 71°32′59.91″E, elev. 2077 m, exp. 0- S, incl. 2–10°, 16 Jul 2021, wp. 1480, M. Nobis, E. Klichowska s.n. (KRA 594031-594033, 594044-594046, 594057-594061); East Tajikistanian (A subregion), Kyzylsu River Valley, S of Alga settl., ca. 14 km NE of Damburacha, steppe on the hill, 39°19′39.87″N / 71°32′49.04″E, elev. 2102 m, exp. 0- S, incl. 2–15°, 16 Jul 2021, wp. 1480 (200 m to the west), M. Nobis, E. Klichowska s.n. (KRA 594862);
KYRGYZSTAN: Naryn Region, Central Tian Shan, ca. 9.5 km W of Naryn, ca. 153 km E of Kazarman, steppe, 11 Jul 2022, elev. 2082 m, 41.437028 N / 75.860948 E, wp 1591, M. Nobis, E. Klichowska, A. Wróbel s.n. (KRA)
Taxonomic notes
The species has been recently described from Turkmenistan (Nobis et al., 2020), and it is a result of hybridization between Stipa sareptana and S. caucasica. Stipa × balkanabatica is the most similar to S. consanguinea Trin. However, it differs well by having more robust, longer (11–15 cm vs. 7–11 cm) awns, longer flowers (10–12 mm vs. 7–9 mm long), longer glumes (32–40 vs. 24–30 mm) as well as the character of vegetative leaves (distinctly scabrous due to densely distributed spinules and prickles vs. glabrous or slightly scabrous due to scattered spinules respectively). However, opposite to typical specimens of S. balkanabatica, the specimens found in Tajikistan and Kyrgyzstan have entirely glabrous and smooth vegetative leaves, what is probably a result of the predominance of S. caucasica genes. Such specimens with glabrous leaves can be distinguished as S. × balkanabatica var. alaiaensis M. Nobis & Klichowska, var. nov. (diagnose: from the typical variety differs in having glabrous and smooth, not distinctly scabrous leaves of vegetative shoots; type: Kyzylsu River Valley, S of Alga settl., ca. 14 km NE of Damburacha, steppe on the hill, 39°19′43.48″N / 71°32′59.91″E, elev. 2077 m, exp. 0- S, incl. 2-10°, 16 Jul 2021, wp. 1480, M. Nobis, E. Klichowska s.n.; holotype, KRA 594031; isotypes, KRA 594032-594033, 594044-594046, 594057-594061; Figure S6 in the Supplementary material).
Distribution and habitats
Stipa × balkanabatica var. alaiaensis occurs in steppe grasslands, in sunny habitats dominated by grasses. In Tajikistan, it was found on hills within the Kyzyl-Suu River valley (Figure 11), within steppes dominated by S. caucasica subsp. nikolai, S. sateptana, S. margellanica, S. arabica, S. hohenackeriana, S. lingua, and others. Whereas in Kyrgyzstan, it grows on the left slope of the Naryn River valley within feathergrass steppes dominated by S. sareptana, S. caucasica subsp. nikolai and S. macroglossa subsp. macroglossa (nomenclature after Nobis et al., 2020). Probably in both countries, the species is more frequent, and subsequent localities are expected to be found.
Figure 11
Stipa × balkanabatica var. alaiaensis M. Nobis & Klichowska on the steppes in Kyzylsu River Valley, S of Alga settl. in Tajikistan (photo. M. Nobis, 2021).

Symphyotrichum cordifolium (L.) G.L.Nesom (Asteraceae)
Synonyms: Aster cordifolius L.
Contributors – Arkadiusz Nowak, Sylwia Nowak
New records
POLAND: Prószków near Opole - the Arboretum in Pomologia, N 50.592943, E 17.882881, elev. 186 m, CF0560 (ATPOL grid); Zimnice Małe, N 50.57001, E 17.928786, elev. 180 m, CF0584 (ATPOL grid); Opole - Wójtowa Wieś, N 50.646459, E 17.925321, CF0503 (ATPOL grid); 5 Oct 2022, A. Nowak, S. Nowak s.n. (OPUN, KRA).
Taxonomic notes
Symphyotrichum cordifolium was first described as Aster cordifolius L. The species belongs to the sect. Symphyotrichum and subsect. Heterophylli, which includes ten taxa native to North America (Brouillet et al., 2006), one of which (Symphyotrichum laeve (L.) Á. Löve & D. Löve) is known in Poland (Euro+Med, 2006). Symphyotrichum cordifolium is a colonial and cespitose plant with basal and proximal leaf bases usually deeply cordate and leaf margins usually sharply or coarsely serrate. Additionally, Symphyotrichum cordifolium has heads (20–300+) in densely paniculiform arrays, involucres cylindro-campanulate to cylindric, and ray laminae (5–)6–8(–10) × 1.4–1.8 mm (Brouillet et al., 2006).
Distribution and habitat
The native range of Symphyotrichum cordifolium comprises the eastern states of USA and southeast Canada (POWO, 2022). According to GBIF (2022), the species was introduced to Norway, Great Britain, the Netherlands, Bavaria in Germany, Sweden, and New Zeeland. It was also assessed as a neophyte plant in the Czech Republic (Pyšek et al., 2012). In Poland, the species is known as a cultivated plant but has never been recorded in natural habitats.
A huge population of the species was found in the naturalistic park of the former Royal Pomological Academy in Prószków near Opole in October 2022. Its size is uncountable and certainly much larger than years ago, when the species was not noticed (Figure 12). Symphyotrichum cordifolium most likely appeared in the Prószków arboretum at the time when the horticultural institute was operating there. Perhaps the plant was acclimatized here for ornamental purposes. However, in the last 30 years, despite very frequent visits by botanists from the Opole University, it has never been spotted in the wild part of the park. Currently, the species occupies almost the entire forest floor and outcompetes even strong competitors that tightly cover the undergrowth, such as Hedera helix. In addition, two smaller species populations were found in oak-hornbeam and riparian stands in Zimnice Małe and Opole-Wójtowa Wieś. As both localities are located in the Oder River valley, there is a high risk of the species spreading along the river.
Figure 12
Symphyotrichum cordifolium (A) in the naturalistic park (B) of the former Royal Pomological Academy in Prószków (photos: A. Nowak (A) and M. Nobis (B) 2022).

In Prószków, the population of S. cordifolium was accompanied by Carpinus betulus, Quercus robur, Q. petraea, Robinia pseudoacacia in the tree layer, Cornus sanguinea, Corylus avellana, Euonymus europaeus in the shrub layer and Aegopodium podagraria, Carex sylvatica, Galanthus nivalis, Geranium robertianum, Hedera helix, Impatiens noli-tangere, Lamium album, L. galeobdolon, L. maculatum, Maianthemum bifolium, Milium effusum, Poa nemoralis, Pulmonaria obscura in the herb layer (nomenclature of species after Euro+Med, 2006).
Thalictrum alpinum L. (Ranunculaceae)
Contributors: Sławomir Wróbel, Anna Wróbel
New record
POLAND: Western Carpathian Mts, Tatra Mts, Mała Łąka Valley, Niżnia Świstówka, slope under Mnichowe Turnie, elev. ca. 1620 m, exp. W, snowbed (Salicetum retuso-reticulatae) and alpine grasslands (Seslerion tatrae) communities on limestone, DG5977 (ATPOL grid), 21 Jul 2022, S. Wróbel s.n. (KRA).
Taxonomic notes
Thalictrum alpinum is a small, glabrous plant reaching up to 15 cm high, making it the smallest representative of the genus Thalictrum in the Tatra Mts. Two other species, Thalictrum minus L. and Thalictrum aquilegiifolium L. are much taller. Moreover, T. alpinum produces only basal 2-ternate leaves, without stipels, and forms sparse flowers organized in a simple raceme inflorescence (Figure 13). On the other hand, T. minus and T. aquilegiifolium produce cauline leaves and form compound inflorescences (Pawłowski, 1956; Tutin et al., 1964). Based on these morphological characters, T. alpinum is well-distinguished from other Thalictrum species occurring in the Tatra Mts.
Figure 13
Thalictrum alpinum in the Niżnia Świstówka in the Western Tatra Mts, Poland: (A) habitat under limestone rock wall; (B) generative shoot; (C) simple raceme fruit-bearing inflorescence (photo. S. Wróbel, 2022).

Distribution and habitat
Thalictrum alpinum is a circumboreal species occurring in northern Eurasia, Alaska, northern Canada, and Greenland, as well as in isolated localities in the mountain regions southwards (Meusel et al., 1965). The species grows in the tundra biome, on rocky habitats and moraines near glaciers, within alpine grasslands, dry alpine meadows, fens, and mires (Newskij, 1937). In Europe, the species is currently observed in the arctic and subarctic zone as well as in mountain ranges: the Sierra Nevada, the Pyrenees, the Alps, the Dinaric Alps, the Carpathians, and in the Caucasus (Meusel et al., 1965). In the Carpathians, T. alpinum was noted in the southern part of the range on several localities between 1500–2250 m a.s.l. in the Bucegi Mts, Romania (Beldie, 1967), while XIX-century records from the eastern part (Rodna Mts and the Ceahlău Massif, Romania) were not confirmed later (Dihoru & Negrean, 2009). In Poland, the species was reported as a fossil component of the Pleistocene flora – fruits of T. alpinum were found in the sediments dated back to the North Polish Glaciation, collected in multiple localities in Poland, including Grel near Nowy Targ, ca. 26 km northwards of the Tatra Mts (Kucowa, 1985), which is the closest locality to the record described here.
During the field research on 21 July 2022 in the Western Tatra Mts, the species was found in one locality within the Tatra National Park, Poland – in the upper part of the Mała Łąka Valley, in the Niżnia Świstówka, on a slope with western exposure at ca. 1620 m a.s.l. under a limestone rock wall (Figure 13). The shoots of T. alpinum were scattered throughout the area of ca. 100 m2 in two different phytocenoses – within snowbed community Salicetum retuso-reticulatae Br.-Bl. 1926 under the rock wall while further downwards of the wall within limestone alpine grasslands of Seslerion tatrae Pawł. 1935. The size of the population was estimated at several hundred individuals in total, with high occurrence frequency in both habitat types. However, only 36 plants formed generative shoots. Moreover, fruit-bearing specimens were restricted exclusively to the snowbed habitat, while in alpine grassland communities, only vegetative shoots appeared. In the middle of the locality, the Braun-Blanquet phytosociological plot was established – on 25 July 2022, 25 m2, elev. 1620 m, exp. W, incl. 45°, herbaceous plant coverage in layer C – 90%, mosses coverage in layer D – 80%, number of herbaceous plant species – 27: Salix reticulata 4, Carex firma 2, Dryas octopetala 2, Pedicularis oederi 2, Swertia perennis 2, Anthoxanthum alpinum 1, Bartsia alpina 1, Carex sempervirens subsp. tatrorum 1, Helianthemum nummularium subsp. grandiflorum 1, Saxifraga moschata 1, Thalictrum alpinum 1, Campanula polymorpha +, Cerastium tatrae +, Crepis jacquinii +, Minuartia verna +, Parnassia palustris +, Picea abies +, Pinguicula alpina +, Poa alpina +, Polygonum viviparum +, Ranunculus alpestris +, Saxifraga aizoides +, S. paniculata +, Scabiosa lucida +, Selaginella selaginoides +, Tofieldia calyculata +, Trisetum alpestre + (nomenclature of the species is given after Mirek et al., 2020).
The described locality of T. alpinum is the first in the Tatra Mts and the first contemporary observation of the plant throughout the Western Carpathians and in Poland. Due to very restricted distribution and a small number of mature individuals (<50), T. alpinum should be regarded as critically endangered (CR) in both the Western Carpathians and Poland according to IUCN 2012 – CR, criterion D (IUCN, 2012).




