Introduction
The family Ranunculaceae is one of the earliest diverging lineages among the eudicots (Stevens,2001), and might have radiated within Ranunculales 121–114 million years ago (Mya) (Anderson et al.,2005), or as early as 125.8–123.0 Mya, as proposed by the “accelerated angiosperm evolution” hypothesis (W. Wang et al.,2016). In Ranunculales, additional evidence from phylogenetic analyses of MADS‐box genes supports whole-genome duplication early in the diversification of angiosperms (Landis et al.,2018; Tank et al.,2015). The split between Aconitum and other genera occurred 24.7 Mya (Park et al.,2020), the divergence between Aconitum L. subgen. Aconitum and subgen. Lycoctonum (DC.) Peterm. has been dated to have occurred approximately 8.24–20.7 Mya (L. Wang et al.,2009), and lastly, Aconitum subgen. Aconitum began to radiate between 2.5 and 6.4 Mya (L. Wang et al.,2009).
Aconitum and Delphinium L., the latter including Consolida Gray, Aconitella Spach, and Staphisagria J. Hill, form the monophyletic tribe Delphinieae Schröd., subtribe Delphiniinae Benth. & Hook (Jabbour & Renner,2011; Keener et al.,1999; M. Tamura,1993; Turland & Barrie,2001; W. Wang et al.,2009). Zygomorphic flowers and presence of diterpene alkaloids have been identified as synapomorphies within this taxonomic group (Jabbour et al.,2009; Johansson,1995). Aconitum consists of the following subgenera: subgen. Aconitum, subgen. Fletcherum (Tamura) Y. Hong & Q. E. Yang, subgen. Galeata (Rapaics) Y. Hong & Q. E. Yang, and subgen. Lycoctonum (Hong et al.,2017; Jabbour & Renner,2011; Kita et al.,1995; Luo et al.,2005; Utelli et al.,2000). The taxonomic rank of subgen. Anthora (Rapaics) Peterm. is unclear and requires further investigation (Novikoff & Mitka,2015).
The genus Aconitum (monkshood) consists of ca. 300 species distributed across the temperate regions of the Northern Hemisphere, with a center of diversification recognized in Eastern Asia (Himalaya, Southwestern China, and Japan) (Kadota,1987; Liangqian & Kadota,2001; Luo et al.,2005). The subgenus Aconitum includes more than 250 species, of which 22 native species (excluding numerous hybrid species) can be found in Europe, a secondary center of Aconitum diversification (Table 1) (Götz,1967; Mitka,2003; Mitka & Starmühler,2000; Novikoff & Mitka,2011,2015; Novikoff et al.,2016; Seitz,1969; Starmühler & Mitka,2001). Recently, efforts have been made to clarify the taxonomic sectional divisions of subgenus Aconitum in the Carpathian Mts, using cytogenetic criteria (see Table 1) (Ilnicki & Mitka,2009,2011; Joachimiak et al.,1999; Mitka et al.,2007). Eight species belonging to this subgenus occur exclusively in the Carpathian and Balkans Mts.
Table 1
Sectional divisions of Aconitum subgen. Aconitum in Europe (Mitka et al.,2017).
Phylogenetic relationships within A. subgen. Aconitum in Europe have not yet been analyzed and remain unknown. Only a few European accessions were included in a genus-wide phylogenetic study, namely A. napellus L. and A. variegatum L. (Luo et al.,2005). Jabbour & Renner (2011) and Xiang et al. (2017) estimated a split between the European accessions A. pentheri Hayek and A. napellus L. to have occurred ca. 0.9 Mya. Thus, very few European accessions were examined, and insufficient geographical sampling did not allow any relevant interpretation.
Aconitum L. subgen. Aconitum is known for its high morphological plasticity and extensive interspecific hybridization (Kita & Ito,2000; Krzakowa & Szweykowski,1976; Sutkowska et al.,2013; Sutkowska, Boroń, et al.,2017; Sutkowska, Warzecha, & Mitka,2017; Zieliński,1982,1982), and the latter is considered to be a major cause of taxonomic unclarity (Kadota,1981; Tutin et al.,1993). Various Aconitum species have been found to possess identical chloroplast DNA (cpDNA) sequences, resulting from horizontal gene transfer (Kita & Ito,2000; Kita et al.,1995; Luo et al.,2005; Utelli et al.,2000). In particular, Aconitum species may contain multiple versions of the nuclear-encoded plastid genes (e.g., rpl32 paralogs), thus exhibiting phylogenetic incongruence (Park et al.,2020).
In a preliminary study, we found two cpDNA haplotypes in A. subgen. Aconitum from the Carpathian Mts that generally fit the cytogenetic (diploids vs. tetraploids) and taxonomic sectional division (sect. Cammarum vs. sect. Aconitum) (see Mitka et al.,2016) criteria.
Here, we aimed to resolve the complicated genetic relationships among the Aconitum taxa throughout its European range, using plastid (cpDNA) and nuclear DNA (internal transcribed spacer, ITS) sequences of species distributed across Western, Central, and Southern Europe, and in the Caucasus Mts. The primary purpose of our phylogenetic analyses was to demonstrate the relationships between the studied species and regions; thus, the pattern of clades retrieved here should not be used solely as a justification for taxonomic decisions (Hörandl,2006).
Taking the differences in the cytogenetic and ecological profiles of the European and Caucasian Aconitum into consideration, we attempted to determine if: (i) the European tetraploids originated in situ from the diploid genetic stock, and (ii) genetic signatures exclusive to diploid and tetraploid species exist, using phylogenetic analyses based on ITS and cpDNA sequences (trnL(UAG)-ndhF region).
Material and Methods
The Study Taxon
The subgenus Aconitum L. in Europe consists of the (i) tetraploid sect. Aconitum [2n(4x) = 32], (ii) diploid sect. Cammarum DC. [2n(2x) = 16], (iii) monospecific sect. Angustifolium (Seitz) Rottensteiner, represented by allopolyploid A. angustifolium Rchb. [2n(6x) = 48], and (iv) triploid nothosect. Acomarum Starm. [2n(3x) = 24]. Sect. Aconitum consists of subsect. Aconitum and subsect. Burnatii Rottensteiner, with the latter possessing a glandular indumentum, which is unusual within the tetraploids (Table 1) (Starmühler & Mitka,2001). In Europe, 10 species belonging to sect. Aconitum, seven to sect. Cammarum, and one to sect. Angustifolium have been noted (Table 1). Intersectional hybrids (A. sect. Aconitum × A. sect. Cammarum) are circumscribed within the nothosect. Acomarum Starm., and consist of seven nothospecies and three hybrid formulae (Starmühler,2001; Wacławska-Ćwiertnia & Mitka,2016).
The tetraploid Aconitum sect. Aconitum encompasses high-mountain species of the subalpine and alpine zones (Table 1) (Ilnicki & Mitka,2009; Mitka,2000,2002; Novikoff & Mitka,2011; Seitz,1969; Sutkowska, Warzecha, & Mitka,2017). The diploid A. sect. Cammarum includes lowland and montane species (up to ca. 1,150 m above sea level) growing in forest environments (Ilnicki & Mitka,2011; Joachimiak et al.,1999; Mitka,2003).
The chromosome numbers were investigated using specimens from the Carpathian and Sudetes Mts (Ilnicki & Mitka,2009,2011; Joachimiak et al.,1999; Mitka et al.,2007), or obtained from the on-line DCBD database (Simon et al.,1999), which is a conspect of chromosome numbers in the tribe Delphinieae (Bosch et al.,2016).
Both these sections (diploids and tetraploids) differ in their nuclear 2C DNA contents (ca. 11 pg vs. 21–22 pg, respectively) (Joachimiak et al.,2018).
Taxon Sampling
The present study included 64 accessions representing A. subgen. Aconitum in Europe, all of which were sequenced for the first in this study. These accessions were as follows: sect. Cammarum: A. degenii Gáyer ssp. degenii (one accession), A. d. ssp. intermedium (Zapał.) Mitka (one), A. d. ssp. paniculatum (Arcang.) Mucher (two), A. d. ssp. rhaeticum Starm. (one), A. ×hebegynum DC. (A. degenii × A. variegatum) (one), A. lasiocarpum Rchb. ssp. kotulae (Pawł.) Starm. & Mitka (one), A. l. ssp. lasiocarpum (one), A. ×pawlowskii Mitka & Starm. (A. lasiocarpum × A. variegatum) (three), A. toxicum Rchb. spp. toxicum (four), A. pilipes (Rchb.) Gáyer (two), A.variegatum L. ssp. nasutum (Rchb.) Götz (four), A. v. ssp. variegatum (two), A. vitosanum (one); sect. Aconitum: A. anglicum Stapf (one), A. bucovinense Zapał. (four), A. burnatii Gáyer (one), A. ×czarnohorense (Zapał.) Mitka (A. bucovinense × A. ×nanum) (one), A. firmum Rchb. ssp. firmum (one), A. f. ssp. fissurae (two), A. f. ssp. moravicum Skalický (one), A. maninense (Skalický) Mitka (three), A. ×nanum (Baumg.) Simonk. (A. bucovinense × A. firmum) (one), A. napellus Rchb. ssp. napellus (one), A. nevadense Gáyer (one), A. pentheri Hayek (two), A. plicatum Rchb. ssp. plicatum (two), A. p. ssp. sudeticum Mitka (four), A. superbum Fritsch (one), A. tauricum Wulfen (one); Nothosect. Acomarum: A. ×cammarum L. em. Fries (A. napellus × A. variegatum), A. ×berdaui Zapał. (A. firmum × A. variegatum).
The Caucasian stock (Luferov,2000) was represented by A. cymbulatum (Schmalh.) Lipsky (one accession), A. nasutum Fisch. ex Rchb. (four), and A. pubiceps Rupr. (one). In total, 27 taxa (species, subspecies, and nothospecies) from the Pyrenees, Alps, Sudetes, Carpathians, and Balkans were included, covering most of the taxonomic variability of A. subgen. Aconitum in Europe (Figure 1, Table S1).
Two accessions from A. subgen. Lycoctonum, i.e., A. lycoctonum L. em. Koelle and A. moldavicum Hacq. (Kita et al.,1995) constituted the outgroup.
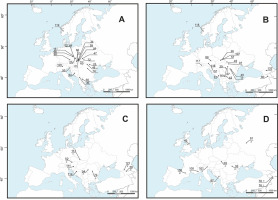
Figure 1
Geographical distribution of the cpDNA haplotypes of Aconitum subgen. Aconitum in Europe and the Caucasus. Haplotype A: Aconitum ×cammarum 01; A. plicatum ssp. sudeticum 02, 2A, 68, 69; A. maninense 09, 23, 66; A. p. ssp. plicatum 16, 98; A. bucovinense 25, 72; A. nanum 32; A. firmum ssp. moravicum 34; A. pentheri 36, 60; A. f. ssp. firmum 47; A. czarnohorense 48; A. degenii ssp. paniculatum 50; A. lasiocarpum ssp. kotulae 59; A. superbum 64; A. ×berdaui 65; A. tauricum 109; A. f. ssp. firmum 119 (A). Haplotype B: A. d. ssp. degenii 07; A. toxicum 17, 49, 61; A. variegatum ssp. variegatum 26; A. bucovinense 29; A. vitosanum 42; A. d. ssp. intermedium 45; A. v. ssp. nasutum 51, 67, 99; A. ×pawlowskii 57, 107; A. toxicum 61; A. nasutum 80, 133; A. pilipes 116; A. d. ssp. rhaeticum 117; A. ×pawlowskii 118 (B). Haplotype C: A. hebegynum 52; A. d. ssp. paniculatum 111; A. ×exaltatum 113; A. v. ssp. nasutum 114; haplotype D: A. f. ssp. fissurae 54, A. bucovinense 74; haplotype E: A. n. ssp. pubiceps 58; A. cymbulatum 121 (C). Ten unique haplotypes – F: A. v. ssp. variegatum 03; G: A. l. ssp. lasiocarpum 33; H: A. firmum 81; I: A. superbum 87; J: A. nasutum 76; K: A. nasutum 78; L: A. burnatii 100; M: A. nevadense 136; N: A. anglicum 46; O: A. napellus 101 (D). For details on accessions origin see Table S1.
DNA Extraction, Amplification, and Sequencing
Recently collected samples (stored as silica-dried leaves) or herbarium specimens of all accessions were obtained (Table S1). Samples for DNA extraction were prepared from these materials, using ca. 2 cm2 of the fully developed leaf blade with no symptoms of damage due to insects or fungal infections. Samples were ground in 2 mL microcentrifuge tubes with three stainless steel beads (φ 3 mm) by shaking in an oscillation mill (MM 200-Retsch, Germany) for 4 min at 25 Hz. DNA was then extracted separately for each sample with Genomic Mini AX Plant DNA extraction kit (A&A Biotechnology, Poland), according to the manufacturer’s protocol.
Two target fragments were used for phylogenetic reconstruction: a fragment of the maternally inherited cpDNA separating plastid trnL(UAG) and ndhF genes (positioned between sites 115,891 and 114,942 relative to the A. kusnezoffii complete plastid genome), and the biparentally inherited ITS region of the ribosomal RNA gene cluster, a tested marker in Aconitum allowing resolution of the infrageneric phylogeny within the genus (Jabbour & Renner,2011; Kita & Ito,2000; Kita et al.,1995; Luo et al.,2005; Utelli et al.,2000; L. Wang et al.,2009).
Undiluted DNA extracts were used as templates in the amplification of both target sequences: trnL(UAG)-ndhF region: Primers trnL(UAG) – 5′-CTGCTTCCTAAGAGCAGCGT-3′ and ndhF – 5′-GAAAGGTATKATCCAYGMATATT-3′ (Shaw et al.,2007) and ITS region: Primers ITS7A – 5′-GGAAGGAGAAGTCGTAACAAGG-3′ (Sang et al.,1995) and ITS4 – 5′-TCCTCCGCTTATTGATATGC-3′ (White et al.,1990). The reaction mixture contained 1× DreamTaq Green buffer (ThermoFisher Scientific, USA), 3.5 mM MgCl2, 0.08 mM of each dNTP, 0.08 µM of both primers, and 1 µL of DreamTaq DNA polymerase (ThermoFisher Scientific). Amplification was performed in a total reaction volume of 50 µL, using a T100 Thermal Cycler (Bio-Rad, USA) with the following temperature profiles:
For the ITS fragment: 5 min at 94 °C; 25 touchdown cycles of 30 s at 94 °C; 30 s at decreasing annealing temperature (from 62.5 °C in the first to 48 °C in the thirtieth cycle); 1 min at 72 °C; and 20 cycles of 30 s at 94 °C, 30 s at 55 °C, and 1 min at 72 °C; followed by 10 min at 72 °C.
For the trnL(UAG)-ndhF fragment: 5 min at 94 °C; 25 touchdown cycles of 30 s at 94 °C; 30 s at decreasing annealing temperature (from 67.5 °C in the first to 55 °C in the twenty-fifth cycle); 1 min at 72 °C; and 20 cycles of 30 s at 94 °C, 30 s at 55 °C, and 1 min at 72 °C; followed by 10 min at 72 °C.
Successful amplification was confirmed by agarose gel electrophoresis, and positive PCR products were purified using Clean-Up DNA purification kit (A&A Biotechnology). The purified PCR products were used as templates in the sequencing reactions.
The two target regions were sequenced in both directions using the PCR primers. However, due to the greater length and frequent polynucleotide regions, a set of internal sequencing primers was used along with PCR primers to ensure bidirectional sequencing of the entire of trnL(UAG)-ndhF spacer: V1_F – 5′-AGGTTGAGTTATTGGTGGATGA-3′, V2_F – 5′-GTTCGCAAAGAACTGAAGTGAC-3′, V3_F – 5′-TGGATGATAGAATAYATATCAAAATCA-3′ (forward primers), and V2_R – 5′-TTTCCGGATTCACCAGCTCTT-3′ and V3_R – 5′-CGAAAAGCCATTACATTCTTAAA-3′ (reverse primers).
Sequencing was performed using BigDye Terminator v.3.1 Cycle Sequencing Kit (Life Technologies, USA) in a T100 thermal cycler (Bio-Rad) and 3500 Series Genetic Analyzer (Life Technologies), using standard protocols.
Sequence Alignment
Individual sequencing reads were examined carefully and compiled into full contigs with ChromasPro 1.7.6 software (Technelysium, Australia). As a relatively high level of nucleotide ambiguity was detected in the ITS sequences, the two independent reads for each contig had to be compared. A given nucleotide position was deemed ambiguous when two peaks were detected at the same position in the sequencing chromatogram, and the weaker peak was at least one third as high as the stronger peak in both independent reads (Fuertes-Aguilar & Nieto-Feliner,2003). Sequences with ambiguous positions, encoded according to the IUPAC nucleotide code, were used for all downstream analyses. Both ITS and trnL(UAG)-ndhF contigs were aligned using the Clustal W algorithm (Thompson et al.,1994) of the MEGA 6 software package (K. Tamura et al.,2013), followed by manual adjustment.
Phylogenetic Analyses
Separate analyses of the trnL(UAG)-ndhF and ITS data sets produced no significant topological discordance for incongruent nodes with Bayesian inference (BI) and maximum likelihood (ML) bootstrap proportions >70%, and the datasets were therefore concatenated, yielding a matrix of 1,531 characters and 16 accession combinations (haplogroups), plus two accessions of the outgroup (Table 2). Substitution model parameters were estimated separately for each partition, using the GTR+G model (with four rate categories) for both the trnL(UAG)-ndhF and ITS regions. The model was selected by FindModel (https://www.hiv.lanl.gov/content/sequence/findmodel/findmodel.html), which uses the ModelTest script (Posada & Crandall,1998).
Table 2
Sixteen haplogroups (based on cpDNA haplotypes and ITS ribotypes) of Aconitum subgen. Aconitum in Europe and the Caucasus (including the 10 unique haplotypes) used in the phylogenetic analyses and the accessions within each haplogroup (species codes are given in Figure 1 and Table S1).
Tree searches were based on a BI method (Rannala & Young,1996) implemented in MrBayes v.1.10.4 (Huelsenbeck & Ronquist,2001; Ronquist et al.,2005). The analysis was carried out by sampling every hundredth generation for 5 million generations, starting with a random tree. The first 1,250 million generations were excluded as burn-in after convergence of the chains, which was evaluated by the average standard deviation of the splitting frequencies below 0.01.
The ML analysis was performed for the concatenated data set in PhyML 3.0 (Guindon et al.,2010). The GTR model of nucleotide substitution was used. Parametric bootstrap values for ML were based on 400 replicates.
DNA sequences were also analyzed using the maximum parsimony (MP) optimality criterion (Felsenstein,2004; Fitch,1971) in PAUP* version 4.0.b10 (Swofford,2002). A heuristic search was conducted with random addition, tree bisection-reconnection (TBR) branch swapping, and the MULTREES option on. The consistency index (CI) and retention index (RI) were calculated with PAUP*, excluding uninformative characters. The strict consensus tree and support for its branches were evaluated by bootstrapping (BS) (Felsenstein,2004), with 174 bootstrap replicates, each with 10 random stepwise additions performed using the same settings as above, and no more than 100 trees were retained per replicate.
Molecular Clock Analyses
We used TempEst v.1.5.1 to test the clock-like behavior of the concatenated dataset (Rambaut et al.,2016). Divergence dating was performed in Beast v.1.10.4 (Drummond et al.,2006; Drummond & Rambaut,2007), which employs a Bayesian Markov chain Monte Carlo (MCMC) approach to coestimate topology, substitution rates, and node ages. All dating runs relied on the GTR+G model, a Yule prior, with uncorrelated and log-normally distributed rate variation across branches.
Several estimations of the divergence time between the subgenera Aconitum (ingroup) and Lycoctonum (outgroup) are available, considering the lack of any reliable Neogene Aconitum fossils. All these estimates were based on the generally accepted substitution rates, and served as secondary calibration points in Beast MCMC analyses, verified by cross-validated calibration approaches (Jabbour & Renner,2011). Jabbour and Renner (2011) estimated the split between A. subgen. Aconitum and A. subgen. Lycoctonum at ca. 11.49, Park et al. (2020) at 11.9, and Xiang et al. (2017) at 15.13 Mya. We considered the age at the divergence of the subgenera as 11.9 Mya (Park et al.,2020) for our analyses.
The MCMC algorithm was run for 3 million generations (25% burn-in), with sampling at every thousandth generation and normal prior distributions and standard deviations of 3 Mya. Tracer v.1.7.1 (Rambaut et al.,2014) was used to confirm acceptable mixing, likelihood stationarity of the MCMC chain, and adequate effective sample sizes for each parameter (>200). The minimum clade credibility tree and associated 95% highest posterior density distributions around the estimated node ages were computed using TreeAnnotator v.1.7.5. The constructed trees were visualized with FigTree v.1.4.3 (2016).
Phylogenetic Networks
To visualize genealogical relations among the cpDNA haplotypes, we used the TCS algorithm of Clement et al. (2000), implemented in POPART software (Leigh & Bryant,2015). It is based on the concept of statistical parsimony and aims at producing an unrooted haplotype phylogenetic network, in which two haplotypes are joined by an edge only if the “probability parsimony” exceeds 0.95 for that edge (Huson et al.,2010).
Results
Characterization of Nucleotide Data
The aligned ITS matrix included 18 unique sequences (16 ingroup + two outgroup) and a total of 632 positions, of which 557 were constant, 60 (9%) were parsimony-informative, and 15 were parsimony-uninformative.
For the cpDNA trnL-ndhF region, the matrix of 18 unique sequences contained a total of 899 positions, with 836 constant, 43 (5%) potentially parsimony-informative, and 20 parsimony-uninformative positions.
The combined (cpDNA + ITS) matrix consisted of 18 unique sequences and 1,531 positions, including 1,408 constant, 35 (2%) potentially parsimony-informative, and 88 parsimony-uninformative positions. Further information on the datasets and tree statistics from MP analyses of the nuclear and chloroplast regions and concatenated data is summarized in Table 3.
Table 3
Dataset and tree statistics from separate and combined analyses of nuclear (ITS) and chloroplast (cpDNA) regions, including the two outgroup accessions.
| ITS | cpDNA trn-ndhF | Combined ITS + cpDNA | |
| Sequences (n) | 18 | 18 | 18 |
| CI of MPTs | 0.8989 | 0.9412 | 0.8889 |
| RI of MPTs | 0.9167 | 0.9434 | 0.9006 |
| Number of MPTs | 224 | 9 | 280 |
| Length of MPTs | 91 | 51 | 144 |
Chloroplast DNA Variation and Geographic Distribution
The 64 accessions of Aconitum subgen. Aconitum (excluding the outgroup accessions) could be categorized into five cpDNA haplotypes, i.e., haplotype A (24 specimens), B (19), C (four), D (two), and E (two), whereas the remaining 10 sequences were unique (Figure 1A–D, Table 2). The trnL(UAG)-ndhF region could not be amplified in three accessions, namely accessions 73, 79, and 108 (Table 2). Haplotype A was distributed across Europe, hapl. B in Europe and the Caucasus, hapl. C was absent in the Carpathians but present in the Alps, Sudetes, and West Balkans, and hapl. D and hapl. E occurred exclusively in the South Carpathians or the Caucasus, respectively (Figure 1).
Table 4 and Table 5 summarizes the site variations within the cpDNA haplotypes. The most conspicuous genetic feature were the unique indels (sites 540–544 and 602–603) that distinguished the diploids from the tetraploids. In this context, the Caucasian accessions appeared to correspond to the tetraploids. They shared indels 602–603 and 648–654 with the tetraploid group, excluding the A. burnatii/nevadense group. A. burnatii and A. nevadense could be distinguished by a substitution at site 505, and from the diploids and tetraploids by indels 540–544 and 648–654, respectively (Table 4). The Caucasian group was heterogeneous. Haplotype K could be identified by indels 612–616 and 723–731, hapl. J by indels 807–821, and hapl. E by sites 781 and 602–603. In comparison, the diploid group was relatively uniform, and point mutations were noted at sites 36, 91, and 118 and indels at sites 328, 333–340, and 807–821 (Table 4 , Table 5).
Table 4
Annotation of the plastid DNA generic spacer trnL(UAG)-ndhF in Aconitum subsp. Aconitum in Europe and the Caucasus, sites 32–616.
Table 5
Annotation of the plastid DNA generic spacer trnL(UAG)-ndhF in Aconitum subsp. Aconitum in Europe and the Caucasus, sites 627–920.
Nuclear ITS Genotype Variation
We observed extremely low nucleotide variation in Aconitum ITS sequences. The 64 accessions (excluding two of the outgroup) were arranged in two ITS ribotypes: R1 (51 specimens) and R2 (two specimens); the remaining seven sequences represented specific ribotypes (ribotypes R3–R9; Table 2). The ITS region of four accessions, namely 02, 46, 50, and 101, could not be amplified. R1 was distributed across Europe and Caucasus, and R2, R6, and R7 occurred only in the Caucasian Mts (Table 2).
Phylogenetic Analysis
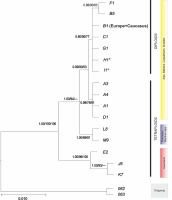
The BI tree, based on the combined DNA plastid and ITS dataset arranged into 16 haplogroups (Table 2), is presented in Figure 2. It suggested the basal, statistically supported position of the Caucasian species (haplogroup E), A. nasutum 76 (haplg. J6), and A. nasutum 78 (haplg. K7) to the European clade. Among the core European clades, two species from the Maritime Alps/Pyrenees, namely A. burnatii 100 (haplg. L8) and A. nevadense 136 (haplg. M9), formed a sister group to the remaining species, with high BI (1.00) and ML bootstrap support (94%) values. The core of the European species was divided into two clades, i.e., the diploid (0.80 BI, 90% ML, 77% MP) and tetraploid species (0.99 BI, 79% ML, 91% MP). Two tetraploids [A. firmum 81 (haplg. H1) and A. superbum 87 (haplg. I1)] were included in the diploid clade. This clade also included haplogroup B1, consisting of both European and Armenian/Caucasian species (Figure 1B). A sister group with moderate support (0.88 BI, 0.90%, ML, 63% MP), constituted of two species, namely A. variegatum 03 (haplg. F1) and A. nasutum 99 (haplg. B5), was also included in this clade (Figure 1).
Figure 2
Bayesian inference tree of Aconitum subgen. Aconitum in Europe and the Caucasus based on concatenated cpDNA + ITS data (haplogroups). The numbers above branches indicate a posteriori probability (BI) and percentage bootstrap values for maximum likelihood (400 replications) and minimum parsimony (174 replications), respectively; * – tetraploids. A1 (20 taxa), A3 – A. firmum ssp. fissurae (the Carpathians), A4 – A. pentheri, B1 (18 taxa), B5 – A. variegatum ssp. nasutum (Šumava, Czech R.), C1 (four taxa), D1 (two taxa), E2 (two taxa), F1 – A. v. ssp. variegatum, G1 – A. lasiocarpum, H1 – A. f. ssp. firmum (Russian Upland), I1 – A. superbum, J6 – A. nasutum (Azerbaijan), K7 – A. nasutum (Armenia), L8 – A. burnatii, M9 – A. nevadense, 62 – A. moldavicum, 63 – A. lycoctonum (for haplogroups B1, C1, D1, and E2, see Table 2 and Figure 1).
Haplotype Network
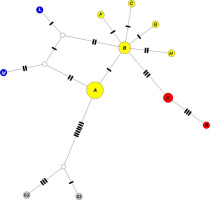
The TCS haplotype network of cpDNA haplotypes (Figure 3) showed a split between the diploid hapl. B and tetraploid hapl. A. Among ingroup haplotypes, those from the Pyrenees/Maritime Alps were genetically the most remote and characterized by intermediate hypothetical haplotypes. These haplotypes, together with hapl. A and B, formed a cyclic node-set, suggesting reticulation. Hapl. B exhibited a star-like pattern, with the other diploid haplotypes radiating out, and linked with the Caucasian species.
Figure 3
Haplotype cpDNA (A–K) + outgroup network obtained from the TCS analysis. The size of the circles is proportional to the frequency of each haplotype. Each bar represents a single mutational change and open circles represent hypothetical haplotypes not observed in this study. Circle colors: blue – Sierra Nevada/the Maritime Alps, yellow – rest of Europe, red – the Caucasus, grey – outgroup. For geographical distribution of haplotypes see Figure 1.
Molecular Clock Estimations
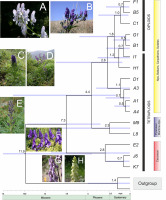
Divergence time estimates for Aconitum in Europe and the Caucasus Mts are shown in Figure 4. The Bayesian analysis showed that the earliest split of the Caucasian genetic stock occurred around 7.3 Mya (Late Miocene). The earliest divergence in Europe was between Aconitum burnatii and A. nevadense, at the Miocene/Pliocene break approximately 4.4 Mya, and the remaining European diploids and tetraploids started to differentiate ca. 2.6 Mya. Diversification within the diploid and tetraploid sections appeared at the beginning of the Quaternary 1.8 Mya and continued till 0.5 Mya (Figure 4).
Figure 4
Molecular clock chronogram of Aconitum subgen. Aconitum in Europe and the Caucasus Mts based on cpDNA + ITS concatenated sequence data (haplogroups) created using BEAST. Bars indicate the 95% posterior density distribution of the nodes and the horizontal axis shows the divergence time of the lineages in million years. The calibration point was 11.9 Mya, according to Park et al. (2020). Photos (© J. Mitka): A. variegatum, Muranska planina, Slovakia (A); A. lasiocarpum, West Bieszczady Mts, East Carpathians, Poland (B); A. napellus, Alps, Dolomites, Italy (C); A. superbum, Dinaric Mts, Bosnia and Hercegovina (D); A. burnatii, Alpes Maritimes, Italy (E); A. nasutum, Transsilvania, Romania (F); A. moldavicum, West Bieszczady Mts, Poland (G); A. lycoctonum, Beskids Mały, West Carpathians, Poland (H). For haplogroups, see Table 2 and Table S1.
Discussion
Geographic-Historical Background
The occurrence of Aconitum in Central Europe can be traced back to as early as the Late Miocene, as suggested by the Aconitum pollen deposits found in the Central Paratethys realm (Central Europe) (Stuchlik & Shatilova,1987). The Caucasian and European lines diverged in the Late Miocene, and internally diversified mainly in the Quaternary, similarly to Ranunculus s. s. (Paun et al.,2005), Syringa (Kim & Jensen,1998), and Wulfenia (Surina et al.,2014), highlighting the significance of this period for the evolution of the European mountain and high-mountain flora.
During the Late Miocene, the temperate forests along the southern coasts of Central and Eastern Parathetys (spread to Western Asia) were continuously replaced by open woodlands. The aridization trend corresponded to forest fragmentation and appearance of open landscapes, the development of grasslands and xerophytic plant communities, and disappearance of subtropical species from the fossil flora (see Dénes et al.,2015; Ivanov et al.,2011). The process was accompanied by a remarkable shift in the composition of fossil mammal assemblages from the Early/Middle Pannonian to the Late Pannonian, reflecting an increase in the seasonality and aridity in the Pannonian Basin area (Harzhauser et al.,2004). The ongoing fragmentation of forests could have disrupted the continuous Aconitum distribution along the southern coast of the Parathetys, contributing to its geographic isolation and evolutionary divergence. This process is illustrated by the Aconitum haplogroup B1 and A. nasutum in the Caucasus and Europe.
The Role of the Caucasus and Central Asia in the European Alpine System
The Caucasus represents a spatial and evolutionary link for many European genera of Asian origin (Ozenda,2009), for example the genera Trollius L. (Després et al.,2003), Acer L. (Grimm & Denk,2014), and Prunus (Volkova et al.,2020). The Asian genetic stock underwent further evolutionary migration to Europe (via the Caucasus and Balkans), i.e., phylogenetic divergence leading to the origin of sister taxa (Bräuchler et al.,2004; Dumolin-Lapègue et al.,1997; Song et al.,2016). The relationships between these regions appear to be older than the Quaternary (Hantemirova et al.,2016). This scenario may have applied to only the diploid line of Aconitum, and the current links between Europe and the Caucasus have been preserved in the diploid cpDNA of haplotype B. In a study on Aconitum in Bela Krajina (Slovenia), Starmühler (1996) discovered a Caucasian species, A. ×tuscheticum (N. Busch) N. Busch (see Luferov,2000), another putative relict of the South European-Caucasian floristic links.
The historical relationships between the Transcaucasia [including Hyrcan and Colchis Tertiary refuges; see Maharramova et al. (2015)] and Europe are well known (Mai,1995). The European Alpine system, the Caucasus, mountains of Central Asia, and stations on the Russian Lowland constitute the Altaic-Alpic geographical subelement, represented by the geographical range of Juniperus sabina L. (Zając & Zając,2009), Saxifraga androsacea L., and Avenula versicolor (Vill.) M. Lainz (Pawłowski,1929). The phylogeographic links between Central Asia and the European Alpine system, especially in Southeastern Europe, are unexpectedly well pronounced in some cases (Kadereit et al.,2008; Ronikier,2011; Winkler et al.,2012).
Independent Evolution of Diploid and Tetraploid Lines
The origin and monophyly of the core European Aconitum subgen. Aconitum remains elusive. Molecular clock analysis dated the split of the tetraploids from the diploid stock at the beginning of the Quaternary (ca. 2.6 Mya). The sister position of the diploid and tetraploid lineages could be misleading, as they could not have originated in situ from a common ancestor and might represent independent genetic lineages in Europe. In this context, A. subgen. Aconitum in Europe could be a nonmonophyletic group.
The simplest “monophyletic” scenario is that the group originated in situ from an ancient, local diploid stock. Molecular analyses did not retrieve any extant diploid species as basal to the tetraploid group in Europe, as was observed for the Japanese tetraploids, where a diploid species, A. volubile Koelle, formed a monophyletic group with all East Asian tetraploid taxa, strongly suggesting it as their ancestral species (Kita & Ito,2000).
However, some extant European tetraploids could have originated in situ from the local, possibly extinct, diploid genetic stock (Mitka et al.,2007), e.g., A. firmum and A. superbum, presently placed in the diploid clade. Their current position among the diploids is probably a relic of their initial diploid state and subsequent tetraploidization or horizontal gene transfer via intersectional hybridization (see below). Whole-genome duplication followed by diploidization in the ancient lineages support the hypothesis of Aconitum palaeoploidy (Park et al.,2020).
Excessive accumulation of 5S rDNA clusters in Aconitum chromosomes (FISH) in the tetraploid species (A. firmum and A. plicatum), followed by a reduction of the basal genome size (Joachimiak et al.,2018), likely occurred during diploidization, which is one of the stages of the cyclical process described as the “wondrous cycle of polyploidy” in plants. It could be a nonrandom process, as suggested by the retention of the original diploid ancestral progenitor genomes (Wendel,2015), at least partially responsible for the paraphyly of the tetraploid and polyphyly of the diploid clades.
Weak support of the European diploid clade and links with the Caucasian genetic stock (haplogroup B1) might indicate its origin from multiple ancestor species that disappeared between 4.4–2.6 Mya. If this is the case, their roots could trace back to arctiotertiary temperate forest elements of Asian origin (Baskin & Baskin,2016; Deng et al.,2015; Popov,1983; Zhang et al.,2014). Some of them disappeared completely, whereas some underwent evolutionary divergence, including genome doubling (tetraploidization), when the global temperatures dropped markedly towards the end of the Pliocene (Abbott,2008; Hultén,1937).
Palaeonedemic Status of Aconitum burnatii/nevadense
Aconitum burnatii and A. nevadense represent the oldest genetic line in Europe, dating back to ca. 4.4 Mya. Their present position at the base of the entire European genetic stock could be a result of their initial diploid status and further palaeoploidisation or speciation by ploidy (see Brochmann et al.,1998; Favarger,1960; Verlaque et al.,1997). According to this hypothesis, both A. burnatii and A. nevadense are autotetraploids, arising from conspecific Tertiary diploid parents, which are now extinct. It may have occurred at the time of the Neogene cooling phases, which culminated in the onset of major glaciation in the Northern Hemisphere (Pearson & Palmer,2000). It is widely accepted that environmental stress resulting from the climatic cooling episodes was the driving force behind the widespread formation of polyploids. These species often occupy habitats different from those of their diploid parents and have been proposed as superior colonizers (Baduel et al.,2018; Soltis & Soltis,2000; Stebbins,1984). This relict group exhibited an independent evolutionary trajectory from the European Aconitum since the Miocene/Pliocene break. Another hypothesis states that the oldest diploid genetic lineage in Europe originated from the extant Central/East Asian diploid species, and this warrants further investigation.
The Pyrenees, Sierra Nevada, and the Maritime Alps, where paleoendemic Aconitum species occur, are one of the most important “cumulative refugial” areas of Mediterranean flora (Aeschimann et al.,2011; Casazza et al.,2005; Médail & Diadema,2009) and fauna (Schmitt,2009) in Europe, representing floristic (Pauli et al.,2003; Väre et al.,2003) and “phylogeographical” (Médail & Diadema,2009) hotspots. The refugial character of the Pyrenees was further confirmed by a phylogeographic study on the subalpine herb Ranunculus platanifolius (Stachurska-Swakoń et al.,2013), where the number of AFLP genetic groups was the highest across the European mountain ranges. The Maritime and Ligurian Alps are believed to be shelters for many Tertiary species, including Saxifraga florulenta Moretti, Silene cordifolia All., Berardia subacaulis Vill., and Viola argenteria B. Moraldo & G. Forneris (see Casazza, Barberis, et al.,2016; Cassaza, Zappa, et al.,2016).
A mechanism underlying the origin of such a pattern could be explained using the example of Silene ciliata Pourret, whose ancestral populations in the Mediterranean Basin might have been forced to migrate northward at the onset of climatic oscillations during the Late Tertiary and the Quaternary periods, resulting in the gradual taxonomic and phylogenetic splitting of the once monophyletic group (Kyrkou et al.,2015).
Reticulation Among European Aconitum
We believe that intersectional hybridization and subsequent genetic introgression are the most relevant factors responsible for the paraphyly of the tetraploid clade (Figure 2) (Mitka et al.,2007,2015; Sutkowska, Boroń, et al.,2017; Sutkowska, Warzecha, & Mitka,2017). Hybridization is frequent in Aconitum (Kita & Ito,2000). Present horizontal transfer of the cpDNA gene between diploid and tetraploid Aconitum species (via reverse “triploid bridge”) has been reported in the Tatra Mts (Sutkowska, Boroń, et al.,2017, Zieliński,1982,1982). Such horizontal gene transfer could be responsible for the observed interchange of cpDNA between the different sections of A. subgen. Aconitum in Europe.
The TCS algorithm showed ancient reticulation among the hypothetical ancestors of the A. burnatii/nevadense group and diploid/tetraploid haplotypes. All these observations indicate the ancient and complicated evolutionary history of the subgenus in Europe, including palaeoploidization, and recent and historical reticulations.
Taxonomic Consequences
The relationships between Caucasian and Balkan/Alpine A. nasutum Rchb. (Götz,1967) remain to be resolved. This species includes both diploid and tetraploid lines (Mucher,1991, Seitz et al.,1972). According to our results, the Caucasian accessions of A. nasutum (76, 78) are tetraploids. Moreover, according to Seitz et al. (1972), A. nasutum from Northeast Turkey is tetraploid. The Transcaucasian-European A. nasutum 051, 080, 099, and 133 (haplotype B) were diploids, based on the marker indel 540–544. Thus, they may belong to different sections, and their taxonomic status should be reevaluated based on their morphological, genetic, and cytogenetic data from Europe, Asia Minor, and the Caucasus. A Caucasian/Asian Minor tetraploid species A. nasutum Fisch. ex Rchb. Il. Acon. 9, 1 (1823) emend. Rupr. Fl. Cauc.: 39 (1869) belonging to sect. Aconitum subsect. Catenata (Steinb. ex H. Riedl) Luferov (2000) was described from the Caucasus [Type: “ad Caucasicum, Herb v. Chamisso!” (Reichenbach,1819); Distribution: Armenia, Iran, Turkey (Davis,1965; Luferov,2000)].
Conclusion
The diploid and tetraploid lines of Aconitum in Europe form independent phylogenies. The links of the European and Caucasian diploid species represented by haplotype B indicate its ancient history in the region and arctiotertiary Asian origins. Paraphyly in the tetraploid clade could have been caused by ancient and present horizontal gene transfer at the section level. High-mountain European tetraploids likely originated from unknown ancestors in the Miocene age, presumably of Asian origin, as early as ca. 2.6 Mya, which is the estimated divergence time for the diploid and tetraploid lines in Europe. Similarly, presumed ancestral diploids, presently extinct, could be ancestral to the extant tetraploid A. burnatii/nevadense line, independent at least since the Late Miocene/Pliocene (4.4 Mya), which may have undergone tetraploidization and evolutionary divergence at least ca. 2.3 Mya.
Handling Editor
Agnieszka Popiela, University of Szczecin, Poland; https://orcid.org/0000-0001-9297-0538


