Introduction
The family Balsaminaceae contains two genera: monotypic Hydrocera and Impatiens, which has over 1,000 species (Fischer, 2004; Gogoi et al., 2018; Grey-Wilson, 1980; Ruchisansakun et al., 2018). The genus Impatiens is characterized by enormous species richness and extremely variable flowers, making it one of the most difficult groups to classify (Bhaskar, 2012; Gogoi et al., 2018; Song et al., 2005). New species of Impatiens continue to be described from different regions of the world (Gogoi et al., 2020; Z. C. Lu et al., 2020; Utami, 2020).
For years, plant species have been solely described based on macroscopic morphological characters (Hooker, 1913; Perrier de la Bathie, 1948; Tardieu-Blot, 1945–1946). Nowadays, an integrative taxonomic approach may help solve some uncertain or complicated cases of classification (Rahelivololona et al., 2018; Ruchisansakun et al., 2015; Shajitha et al., 2016; Yu et al., 2015). The progress in molecular techniques as well as in microscopic imaging using a scanning electron microscope (SEM) constitute additional approaches to determine the boundaries between organisms (Brisson & Peterson, 1976; Godfray & Knapp, 2004). The SEM enables observation and study of small-sized structures that were impossible to examine using conventional light microscopy (Heywood, 1969; Stace, 1992).
Seed shape and seed coat micromorphology are useful for solving taxonomic problems in spermatophytes, including Balsaminaceae (see Cai et al., 2013; W. Chen, Liu, et al., 2007; Utami & Shimizu, 2005; Zhang et al., 2016). However, seed coat micromorphology using the SEM technique has been studied in fewer than one fifth of the Impatiens species (Maciejewska-Rutkowska & Janczak, 2016). Of about 150 balsams known from NE India, seeds of almost 20 were investigated this way, for which, however, material was collected from other distribution sites, and only in one case (I. nagorum; Moaakum et al., 2017) seeds came from NE India. Seed descriptions for Impatiens spp. are not common in literature and often include only macroscopic observations. Such studies are often incomplete and crucial information is missing, e.g., either measurements or only SEM images are published; these are rarely published together (Abid et al., 2011). To date, fewer than 200 species (<20%) have at least one study on seed ultrastructure published (Maciejewska-Rutkowska & Janczak, 2016), and only approximately 20 species (ca. 2%; our unpublished review) of balsams have more than one study available. However, studies have highlighted the importance of seed micromorphology as a useful feature in species identification (Y. Q. Lu & Chen, 1991; Shimizu, 1979) and that seed ultrastructure could be indicative of phylogenetic relationships (Song et al., 2003, 2005; Yuan et al., 2004).
The present study aimed to describe micromorphological characters (based on SEM and biometrical traits) of seeds from nine species of the genus Impatiens collected from Northeast India and provide data useful for solving its taxonomic problems.
Material and Methods
We analyzed seeds from nine species of the genus Impatiens. We collected two−three seeds from three individuals per species during the full fruiting period. The seeds were collected from Northeast India (Table 1). The seeds were sputter-coated with gold and micro-morphological data were studied using an SEM (FEI Quanta 200 SEM and Carl Zeiss EVO 18) at the Central National Herbarium, Botanical Survey of India, Howrah & Botanical Survey of India, ERC, Shillong, Meghalaya. The digital images obtained from the SEM were trimmed and arranged in plates using Corel Draw 2018. The measurements (approximately six−nine for each species) of the seeds (seed length − mm; seed width − mm) were performed based on the SEM pictures of the seeds using the biometric software Image J (Ferreira & Wayne, 2010). The types of seed surface and shape were adapted from Song et al. (2005) and Cai et al. (2013).
Among the nine presented taxa, two (I. anjawensis and I. haridasanii) are newly described (Gogoi et al., 2018; Hareesh & Sabu, 2017) and one (I. nyimana) is a recent addition to the flora of Northeast India (Gogoi et al., 2018; Lidén & Adhikari, 2019). Previously, I. nyimana was only known to occur in eastern Tibet (Y. L. Chen, Akiyama, & Ohba,2007). Impatiens nyimana was included in molecular and morphological studies by Yu et al. (2015); however, its SEM images were not published. Recently, I. mengtszeana, which was previously known to be from China (Y. L. Chen, Akiyama, & Ohba,2007), was synonymized under I. pulchra, a species known from Southeast Asia and Northeast India (Gogoi et al., 2018; Ruchisansakun et al., 2018). Five of the nine species analyzed in the present study have not been studied using the SEM technique before (Table 1).
Voucher specimens of studied taxa were deposited at Botanical Survey of India (CAL), Botanical Survey of India, Eastern Regional Center (ASSAM) & Botanical Survey of India, Arunachal Pradesh Regional Centre (ARUN) (Table 1).
Table 1
Studied Impatiens species.
Taxon | Studied material | Habitat of the species | Remarks |
Impatiens anjawensis Borah, Kandwal, Chhetri & Gogoi | Arunachal Pradesh, Anjaw District, Walong, 4 km from Pimple top towards Walong, 96°58′32.27″ E, 28°10′44.00″ N, 3,690 m, 2016-09-07, S. Borah 34905 (CAL, ASSAM & ARUN) | Moist shady mountain slopes in subalpine forest | Newly described (Gogoi et al., 2018) |
Impatiens decipiens Hook. f.* | Mangan Road, Pakchar, 2017-08-25, R. Gogoi & A. Kumar 73605 (CAL) | Moist hilly slopes and forest margins in subtropical forest | - |
Impatiens drepanophora Hook. f. | North Sikkim, Kabi to Mangan, 1,100–1,700 m, 2017-08-25, R. Gogoi & A. Kumar 73604 (CAL) | Moist forest margins and road sides, also in open areas near streams in subtropical to lower temperate forest | - |
Impatiens haridasanii Hareesh & M. Sabu* | Nagaland, Noklak, N. Odyuio & R. Domari 131936 (CAL) | Moist rocky open areas and in hilly slopes of subtropical forest | Newly described (Hareesh & Sabu, 2017) |
Impatiens jurpia Buch.-Ham. ex Hook. f. & Thomson* | Arunachal Pradesh, Lower Subansiri District, Ziro, Monipuleng to Pine grove, 1,500 m, 2013-08-01, R. Gogoi 30340 (CAL & ARUN) | Moist hilly slopes and in road sides of subtropical primary forests | - |
Impatiens nyimana C. Marquand & Airy Shaw | Arunachal Pradesh, Anjaw District, way to Pimple top from Helmet top, 96°59′34.52″ E, 28°10′4.33″ N, 3,170 m, 2016-09-07, S. Borah & M. K. Kandwal 34907 (ARUN) | Wet alpine meadows | Recently found in India (Gogoi et al., 2018; Lidén & Adhikari, 2019) |
Impatiens pulchra Hook. f. & Thomson* | West Sikkim, Yuksum, Rimbi Waterfall, 2017-09-01, R. Gogoi & A. Kumar 73632 (CAL) | In highly moist areas near streams or waterfalls in subtropical forest | Impatiens mengtszeana was synonymized under I. pulchra (Ruchisansakun et al., 2018) |
Impatiens spirifera Hook. f. & Thomson* | North Sikkim, in between Chungthang to Lachen, 2017-10-05, R. Gogoi 73701 (CAL) | Margins of primary forest and in moist road sides from subtropical to temperate forest | - |
Impatiens sulcata Wall. | North Sikkim, way to Katao, 2017-10-08, R. Gogoi 73710 (CAL) | Open areas in the forest margins and road sides of subalpine forest | Species with wide Himalayan distribution (Y. L. Chen, Akiyama, & Ohba,2007; Gogoi et al., 2018; Grey-Wilson, 1991; Nasir, 1980) |
Results
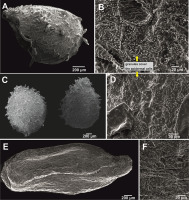
The ultrastructure of the seeds observed under the SEM showed three morphological types: reticulate (four taxa), protrusive (four taxa), and granulate (represented by one taxon; Table 2). Their lengths and widths were different among the taxa and ranged from 1.2 to 3.6 mm and 0.7 to 2.1 mm, respectively. The seeds of the investigated species had ellipsoid, subellipsoid, or subspheroid shapes.
Table 2
Comparison of the morphological characters of the seeds of the analyzed species from Impatiens genus with data from the literature.
Taxon | This study results | Literature data | Source | Figure | |||
Length × Width (mm) | Color | Shape | Ornamentation | ||||
Impatiens anjawensis Borah, Kandwal, Chhetri & Gogoi | 2.0 × 1.3 | Light brown | Ellipsoid | Reticulate type Fine reticulate subtype | 3.0 mm long, 2.5 mm wide; white, ovoid | Gogoi et al., 2018 | Figure 1A,B |
Impatiens decipiens Hook. f. | 1.8 × 1.2 | Light brown | Subellipsoid | Reticulate type Fine reticulate subtype Granules cover the epidermal cells | Not studied | - | Figure 1C,D |
Impatiens drepanophora Hook. f. | 1.4 × 1.1 | Creamy or light brown | Subellipsoid | Protrusive type Digitiform subtype | 2.2–2.9 mm long; 1.3–1.7 mm wide; dark-brown; subellipsoid; protrusive type, digitiform subtype | Song et al., 2005 | Figure 1E,F |
Impatiens haridasanii Hareesh & M. Sabu | 1.4 × 0.7 | Light brown | Ellipsoid | Protrusive type Squamalate subtype Granules cover the epidermal cells | Not studied | - | Figure 2A,B |
Impatiens jurpia Buch.-Ham. ex Hook. f. & Thomson | 2.4 × 2.1 | Greenish or dark brown | Subspheroid | Granulate type | Not studied | - | Figure 2C,D |
Impatiens nyimana C. Marquand & Airy Shaw | 2.5 × 0.9 | - | Subellipsoid | Reticulate type Fine reticulate subtype | Ovoid, corrugate | Yu et al., 2015 | Figure 2E,F |
Impatiens pulchra Hook. f. & Thomson | 1.2 × 0.8 | Greenish or dark brown | Subspheroid | Protrusive type Cristate subtype Granules cover the epidermal cells | Not studied | - | Figure 3A,B |
Impatiens spirifera Hook. f. & Thomson | 2.0 × 1.4 | Grey | Subspheroid | Protrusive type Cristate subtype Granules cover the epidermal cells | Not studied | - | Figure 3C,D |
Impatiens sulcata Wall. | 3.6 × 1.4 | Light brown or cream coloured | Subellipsoid | Reticulate type Fine reticulate subtype | 3.2–4.0 mm long, 1.3–2.4 mm wide; brown; subellipsoid /ovate; areolate subtype/granulate; ovoid, corrugate | Figure 3E,F | |
The reticulate type was characterized by epidermal cells that formed reticulate ornamentation with varied shapes and sizes. This group comprises I. anjawensis (Figure 1A,B), I. decipiens (Figure 1C,D), I. nyimana (Figure 2E,F), and I. sulcata (Figure 3E,F). These species belong to the fine reticulate subtype, with epidermal cells evenly distributed and slightly bulged. The seeds in this category had ellipsoid or subellipsoid shapes and the size was in the range of 1.8–3.6 (length) × 0.9–1.4 mm (width; Table 2). Only one species in this subtype, I. decipiens, had seeds with granules covering the epidermal cells.
Figure 1
Seed general view and coat micromorphology of analyzed balsams from Northeast India. (A–D) Reticulate type: Impatiens anjawensis (A,B), I. decipiens (C,D); (E,F) protrusive type: I. drepanophora.
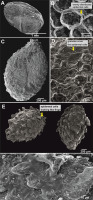
The protrusive type, characterized by some epidermal cells of the seedcoat protruding significantly to form projections of varied shapes, included I. drepanophora (Figure 1E,F), I. haridasanii (Figure 2A,B), I. pulchra (Figure 3A,B), and I. spirifera (Figure 3C,D). In this type, three species had granules covering their epidermal cells (Table 2). Impatiens drepanophora represented the digitiform subtype with epidermal cells looking like fingers. Impatiens haridasanii represented the squamalate subtype having scales covering the seed coat. The last two species in this type, I. pulchra and I. spirifera, belonged to the cristate subtype.
Figure 2
Seed general view and coat micromorphology of analyzed balsams from Northeast India. (A,B) Protrusive type: Impatiens anjawensis; (C,D) granulate type: I. jurpia; (E,F) reticulate type: I. nyimana.

Only one species, I. jurpia (Figure 2C,D), represented the last type, granulate, which was characterized by granulate protrusions evenly covering the entire seed surface.
Discussion
Previous studies on seed sculpture have shown its usefulness in plant taxonomy, as seen for Asteraceae (Shabestari et al., 2013), Caryophyllaceae (Ullah, Papini, et al., 2019; Ullah, Zaman, et al., 2019), Poaceae (Martín-Gómez, Rewicz, Goriewa-Duba, et al., 2019), Ranunculaceae (Hadidchi et al., 2020; Martín-Gómez, Rewicz, & Cervantes, 2019; Rewicz et al., 2017), and Orchidaceae (Ortúñez et al., 2006; Rewicz et al., 2016). Our research is the first insight into the seed ultrastructure of five species from the genus Impatiens from Northeast India. Among the analyzed taxa, differences in seed ultrastructure (especially in I. jurpia, I. pulchra, and I. spirifera) and shape distinguished them from each other. Studies have shown a large variation in the ultrastructure of the seed coat in Impatiens (Abid et al., 2011; Y. Q. Lu & Chen, 1991; Shimizu, 1979; Song et al., 2005) and our results confirmed this high diversity. In the four analyzed species, I. decipiens, I. haridasanii, I. pulchra, and I. spirifera, we found a prominent feature of the sculpture on seed coats, granules on the seed surface, which could be useful for the taxonomy of this genus. Abid et al. (2011) considered this feature as important for the recognition of two subspecies of I. bicolor (I. bicolor ssp. bicolor and I. bicolor ssp. pseudo-bicolor).
However, without testing seeds from a wide spatial and habitat range, we should be careful before considering seeds as taxonomically valid for identification. Further research may provide answers concerning the level of phenotypic plasticity of seeds in the analyzed species. It is necessary to determine whether the seeds of these species have a stable ultrastructure and shape to be useful as a tool in taxonomy. Species with a wide distribution, such as I. sulcata or I. noli-tangere, may be a model for such future studies.
Due to the scarcity of seed biometrics and seed coat ultrastructure data, we could only compare the obtained results to the data available for three species, I. anjawensis, I. drepanophora, and I. sulcata (Table 2). For two species, I. anjawensis and I. drepanophora, biometric analyses showed that our seed length and width values were lower than those described in the literature. Only the seed length of I. sulcata was higher than that in the literature data. Unfortunately, for I. nyimana, the literature data did not include seed measurements (Table 2).
Our results of seed shape and ultrastructure agree with the published data only in the case of I. drepanophora (Song et al., 2005). The seed shape and ornamentation information obtained in our study differs from that of the earlier studies in I. sulcata (Abid et al., 2011; Cai et al., 2013; Yu et al., 2015) (Table 2). For I. drepanophora and I. sulcata, this may indicate high phenotypic plasticity in these species. Therefore, seed characteristics may not be useful in identifying I. drepanophora and I. sulcata. Previous descriptions of the seeds of I. nyimana (Yu et al., 2015) differ from our results (ovoid, corrugate and subellipsoid, reticulate type, and the fine reticulate subtype). Results presented by Gogoi et al. (2018) do not include seed ornamentation information for I. anjawensis.
These differences could be an effect of deformation of seeds during air drying in storage or the collection of immature seeds. In the taxa with wide spatial distribution, this phenomenon could also be an effect of internal variability or even potential cryptic diversity. For example, the micrographs of I. noli-tangere seeds from China and Japan are different in their seed coat ultrastructure (W. Chen, Liu, et al., 2007; Jin et al., 2008; Song et al., 2005).
The most complicated case is I. pulchra, where there is no published data on seed ultrastructure. However, during the recent revision of Myanmarese balsams, Ruchisansakun et al. (2018) synonymized I. mengtszeana, known from China and Thailand, with I. pulchra. Impatiens mengtszeana has published data on its seed ultrastructure (Song et al., 2005; Utami & Shimizu, 2005). If seed shape and ultrastructure is considered as a stable taxonomic feature (Stace, 1992), our I. pulchra micrographs should be similar to the micrographs of I. mengtszeana (= I. pulchra sensu Ruchisansakun et al., 2018) from China (Song et al., 2005; Utami & Shimizu, 2005). However, our study showed different shape and ornamentation of seeds in I. pulchra (seeds of I. pulchra were subspheroid and had the protrusive type of ornamentation, while I. mengtszeana had spheroid seeds and the squamalate subtype of ultrastructure; Song et al., 2005) and the specimens we examined had granules covering their epidermal cells (Figure 3B). Thus, the taxonomic status of these two taxa requires further investigation.
Overall, our observations concerning the ultrastructure of seeds from Impatiens spp. provide knowledge of the diversity in this group.
Handling Editor
Agnieszka Popiela, University of Szczecin, Poland; https://orcid.org/0000-0001-9297-0538
Authors’ Contributions
RG collected material (I. decipiens, I. drepanophora, I. haridasanii, I. jurpia, I. pulchra, I. spirifera, and I. sulcata) as well as initiated SEM imaging of all species; SB collected material (I. anjawensis and I. nyimana); AR, WA, and RG developed research designing; WA performed literature survey; AR, WA, RG, and SB wrote the main manuscript text; AR, RG prepared figures; RG prepared Table 1; AR and WA prepared Table 2