. Introduction
Polystichum setiferum (Forssk.) Moore ex Woynar. is a fern species with an Atlantic and sub-Mediterranean distribution, from Canary Islands, northern Africa to Ireland in the west, to the Balkans and the Caucasus Mountains in the east (Dostál & Reichstein, 1984). Its northeastern limit of distribution in central Europe has been supposed until recently to lie in Hungary and Romania (Dostál & Reichstein, 1984). Although this species was reported from regions close to the Polish-Czech border as early as in the nineteenth century (Milde, 1855, 1865) under different names (e.g., Aspidium aculeatum Sw.), it was regarded not to occur in the Czech Republic and Poland by contemporary floras (Dostál & Reichstein, 1984; Šourková, 1997; Szafer et al., 1988). This was due to the lack of herbarium documentation and similarity of some forms of Polystichum aculeatum (L.) Roth to P. setiferum, leading to frequent misidentification (Dostál & Reichstein, 1984). Nevertheless, a recent detailed morphological study of herbarium materials allowed the identification of P. setiferum in herbarium collections dated 1881–1935 from the territory of the Czech Republic (Ekrt, 2016). These individuals came from Moravskoslezské Beskydy Mountains (Mt Kněhyně and Mt Smrk), Moravský kras (Olomučany), Jeseníky Mountains (Rýmařov, Mt Praděd, Velkákotlina), and Rychlebské hory (Lázně Jeseník) (Figure 1). Currently, P. setiferum has not been confirmed at these locations, and it is regarded as an extinct species in the Czech flora (Kaplan et al., 2017). The species occurs on moderately acidic, humid soil in beech and mixed forests in moderately high mountains with mild winters (Dostál & Reichstein, 1984). Polystichum setiferum is a diploid species (2n = 82), which is one of the parental species of the allotetraploid P. aculeatum (2n = 164) (Dostál & Reichstein, 1984). Therefore, these morphologically similar species can be unequivocally distinguished by chromosome count or nuclear DNA content analysis.
Figure 1
Distribution of Polystichum setiferum at the northeastern limit of its range based on verified herbarium specimens (circles), literature data (triangles), and the present study (square). Historical records were based on Ekrt (2016) and updated from Kaplan et al. (2017).
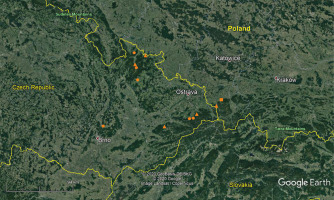
Based on past and recent data, we undertook field surveys for P. setiferum in the Opawskie Mountains and in the western part of the Beskid Śląski mountain range (Silesian Beskid), areas close to the historical locations of the species in the Eastern Sudetes and the Western Carpathians, respectively. The aim of our study was to verify the presence of P. setiferum in Poland in light of the historical data.
. Material and Methods
Field studies aimed at finding P. setiferum in the Opawskie Mountains and Beskid Śląski were conducted between 2018 and 2020. According to our observations, the best season for the search of P. setiferum is winter when all other species besides those of Polystichum are gone.
Analysis of the morphological characteristics, listed in Table 1, was based on data from literature (Dostál & Reichstein, 1984; Rich & Jermy, 1998).
Table 1
Morphological and cytogenetic comparison of Polystichum setiferum and P. aculeatum.
Herbarium studies were performed at the herbaria of the Jagiellonian University (KRA) and the Institute of Botany, Polish Academy of Sciences (KRAM).
Stomatal cell length (n = 100) was determined using leaf fragments placed between glass slides in a water drop, and was measured using a Delta Optical microscope, model Genetic Pro, with an ocular micrometer and a calibration glass slide (Opta-Tech, Poland).
For nuclear DNA content estimation, five leaf samples from one individual of putative P. setiferum and three samples from different P. aculeatum individuals, growing in the neighborhood of P. setiferum, were analyzed on three different days. Nuclei were released simultaneously from fresh leaves a sample species and an internal standard (Allium cepa ‘Alice,’ 34.89 pg/2C; Doležel et al., 1998) by placing chopped leaves in a Petri dish with 1 mL of Galbraith’s buffer (Galbraith et al., 1983) supplemented with propidium iodide (PI; 50 µ g cm−3), ribonuclease A (50 µ g cm−3), and 1% (v/v) polyvinylpyrrolidone (PVP). The suspension was passed through a 50-µ m mesh nylon filter and analyzed using a CyFlow SL Green (Partec GmbH, Germany) flow cytometer equipped with a high-grade solid-state laser with green light emission at 532 nm, long-pass filter RG 590 E, DM 560 A, as well as side (SSC) and forward (FSC) scatters. Analyses were performed on three–four samples of each taxon. For each sample, the DNA content was established in 3,000–5,000 nuclei. Histograms were analyzed using the software FloMax (Partec GmbH). The coefficient of variation (CV ) of the G0/G1 peak of Polystichum sp. ranged between 3.8% and 5.2%. Nuclear DNA content was calculated using the linear relationship between the ratio of the 2C peak positions Polystichum/Allium on a histogram of fluorescence intensities.
. Results and Discussion
Field studies performed during 2018–2020 in Poland, in regions of historical occurrence of P. setiferum, led to the identification of one individual in the western part of the Beskid Śląski mountain range (Figure 1), morphologically corresponding well to this species (Figure 2 and Figure 3). It was found by the first author on December 21, 2019, on the southwestern slopes of Mt Czupel near Brenna, in the western part of the Beskid Śląski mountain range, in one of the abandoned sandstone quarries at ca. 480 m a.s.l. (ATPOL DG0201 square), covered with a 100-year-old beech forest with an admixture of artificially planted larch trees in a half-shaded habitat (Figure 4). This plant was accompanied by several individuals of P. aculeatum located further apart.
Figure 2
Leaves of Polystichum setiferum (right) from Mt Czupel in the Beskid Śląski and P. aculeatum (left) from the Soła Valley in Kobiernice in the Pogórze Śląskie (Poland) (photo by D. Tlałka, January 8, 2020).

Figure 3
Leaf fragments of Polystichum setiferum (top) and P. aculeatum (middle) from Mt Czupel, as well as that of P. aculeatum var. aristatum Christ. (bottom) from Mt Jasieniowa in the Pogórze Cieszyńskie (Poland).

Figure 4
Habitat of Polystichum setiferum at Mt Czupel in the Beskid Śląski (Poland) (photo by D. Tlałka, July 7, 2020).

The identification was based on the investigation of the morphological characteristics distinguishing P. setiferum and P. aculeatum (Table 1). The most pronounced morphological characteristics of P. setiferum differentiating it from P. aculeatum are the relatively long petiole, leaf blade truncated at the base, and stalked, hair-tipped pinnules.
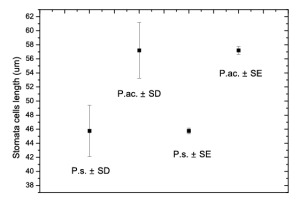
Stomata length or stomatal cell (guard cell) length could also be useful in differentiating the two species (Ekrt, 2016). The results of the stomatal cell measurements of P. setiferum and P. aculeatum from the stand (Figure 5) show that the length of stomatal cells of the putative P. setiferum individual was evidently lower than that of P. aculeatum plants. The length ratio of P. setiferum to P. aculeatum stomatal cells was 0.8, which corresponds well with the stomata length ratio of specimens from the Czech Republic (0.8) (Ekrt, 2016) and those originating from southern Europe (0.76) (Ekrt, 2016).
Figure 5
Length of stomata cells (guard cells) of Polystichum setiferum (P.s.) and P. aculeatum (P.ac.) from the stand in the Beskid Śląski. The means ± SD and ± SE (n = 100) are shown.
In addition to morphological characteristics, the most unequivocal evidence discriminating the two species is the chromosome number count or the corresponding nuclear DNA content measurements. Nuclear DNA content (2C values) of the putative P. setiferum and P. aculeatum growing in the neighborhood, collected from the new stand, were 15.46 and 28.77 pg, respectively (Table 2). To the best of our knowledge, this is the first study to estimate the genome size of these species (Leitch et al., 2019). The results revealed that 2C values of the investigated species differed nearly twofold, corresponding well with their ploidy level (Dostál & Reichstein, 1984) (Table 1). Moreover, the 2C value of P. setiferum found in our studies is the same as that of the diploid American species, P. acrostichoides (Michx.) Schott (15.5 pg/2C) (Bainard et al., 2011). These data indicate that the analyzed P. setiferum individual indeed represents this diploid species and not tetraploid P. aculeatum or its triploid hybrid with P. setiferum, that is, P. ×bicknellii (Christ) Hahne, which should have a 2C value intermediate between those of the parental species.
Table 2
DNA content (pg/2C) of the investigated Polystichum species from Mt Czupel in the Beskid Śląski.
| Species | DNA content (pg/2C) |
|---|---|
| Polystichum setiferum | 15.456 ± 0.070 |
| Polystichum aculeatum | 28.773 ± 0.199 |
An additional search for P. setiferum specimens among those of P. aculeatum collected in Poland in the KRA and KRAM herbaria did not result in finding P. setiferum even though hundreds of P. aculeatum sheets were deposited in these herbaria. This indicates that P. setiferum must have already been a very rare species in the past.
The newly identified, isolated stand of P. setiferum is situated approximately 10 km from the nearest historical location at Wielka Czantoria near Ustroń (Milde, 1855) and nearly 500 km from other existing populations of this species in Austria (Steiermark) (Virtual Herbaria, 2020, search term: “Polystichum setiferum”), southern Hungary (Mecsek Mountains) (Bátori et al., 2009), and western Romania (near Arad) (Virtual Herbaria, 2020, search term: “Polystichum setiferum”).
The present finding of P. setiferum determines its northeastern limit of distribution in Europe, which is a remnant of the more widespread occurrence of this species in the past. However, it cannot be excluded that the discussed stand is a result of a relatively new colonization by spores originating from other presently unidentified or extinct sites located in Poland or the Czech Republic.
As it is the only extant stand of P. setiferum in the area of the Sudetes and the Carpathian Mountains, it deserves special attention and protection.


