. Introduction
To ensure food security for the rapidly growing world population, crop production should be increased by 50% by 2050 (van Dijk et al., 2021). The global temperature is rising continuously and is expected to rise by 5–7 °C at the end of the century, which may affect crop production, especially cereals (Beena et al., 2013; IPCC, 2021). Reduced production of cereals may create a food security concern, particularly in developing countries like Pakistan (Janni et al., 2020; Sarwar et al., 2023). To combat the situation, special attention is needed for better crop productivity in current climatic conditions (Dinar et al., 2019; Khan et al., 2023).
Cereals are the dietary need for a large portion of the population (Waldamichael et al., 2022) in which the rice crop is ranked at a significant position due to its widespread consumption (Ruan et al., 2023). Rice is a dominant crop in more than 30 countries of the world, with above 90 % production in Asia (Kondamudi et al., 2012). High temperature stress is one of the key factors in current global warming situation. Rice crop yields may decline up to 41 % due to elevated temperature (Shah et al., 2011). The rice crop is suffering in the current weather conditions as it is highly sensitive to abiotic stresses, especially at the reproductive stage (Mujtaba et al., 2022; Neang et al., 2020). Elevated temperature (≥32 °C) causes a significant decline in crop growth, stomatal conductance, and pollen viability, which in turn reduces plant biomass and enhances the proportion of sterile grains (Beena et al., 2018; Fu et al., 2012; Jagadish et al., 2012).
Many strategies are being used to manage abiotic stresses, including nutrient application and the use of hormones or growth regulators. Auxin is a very important growth regulator (Barbier et al., 2019; Matthes et al., 2019) improving plant growth and panicle development in normal and stressed environments (Abou El-ghit, 2015; Li et al., 2019). Similarly, it improves the antioxidant activity, which stabilises plants under abiotic stresses (Khan et al., 2023). Likewise, optimum levels of auxin also improve the plant rooting mechanism and enhance nutrient uptake (Kurpea & Smalle, 2020).
In a stressed environment, especially at the reproductive stage, auxin production in plants is reduced, which causes pollen viability and thus reduced fertility in the rice crop (Fu et al., 2015; Zhang & Peer, 2017). Auxin (Indole-3-acetic acid) is naturally produced in plants, while its concentration can also be enhanced through its exogenous application. It has been reported that the application of naphthalene acetic acid (NAA) improves the auxin level in plants (Sarwar et al., 2019), which strengthens leaf chlorophyll contents and other photosynthetic activities in plants (Hossain, 2023). It also plays a crucial role in optimisation of physiological processes like leaf senescence, leaf & fruit abscission, fruit setting, and development of vascular tissues (Alabadí et al., 2009). NAA also improves pollen viability, thereby enhancing the number of fertile grains, leading toward better crop yield and quality (Bakhsh et al., 2011; Hussain et al., 2021; Sajid et al., 2016).
Most plants produce antioxidants in stress conditions to manage the situation, while this process can also be triggered by exogenous application of some growth regulators and essential nutrients. Auxin can also work as a stimulator to produce some antioxidants under drought, heat, or nutritional stress (Al-Duraid et al., 2019; Khan et al., 2023). Eminent results have been achieved with NAA application in horticultural and field crops (Basuchaudhuri, 2016; Fatima et al., 2008; Hossain, 2023; Sarwar et al., 2019). Studies have also explored that NAA application improves rice grain quality in terms of its protein contents, fineness, and moisture contents (Jahan & Adam, 2014). Moreover, exogenous application of auxin mitigates heat stress and improves crop yield and grain quality (Aryan et al., 2023). Therefore, we hypothesised that application of NAA at the flowering stage may mitigate the elevated temperature effect, resulting in improvement of grain quality as well as crop yield and its component. Thus, different available genotypes were grown and observed under various levels of NAA. Our major objective was to determine the optimum level of NAA application at the reproductive stage along with the best responsive cultivar in the current climatic conditions. This study will provide valuable information for farmers to maintain rice yield and quality standards in the current climatic condition.
. Materials and methods
This experiment was conducted in pots placed in a wire house during the 2017 kharif season at the agronomic research area of the Department of Agronomy, Bahauddin Zakariya University Multan, Pakistan. Pots (25 cm × 40 cm × 30 cm) were filled with fertile soil collected from the surrounding field area. Before experimentation, soil samples were collected for the analysis of different physico-chemical properties. The soil was determined as silty clay following the hydrometer technique. Similarly, soil pH (7.8) and EC (1.73 dS m−1) were determined using lab instruments. Moreover, the soil contained 0.46% of organic matter, total Nitrogen (1.8%), Phosphorus (6.52 mg/kg), and available Potassium (175 mg/kg).
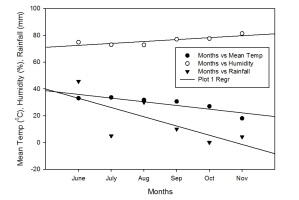
The seeds of rice cultivars were collected from the rice research station Kala Shah Kaku Lahore Punjab, Pakistan. Four (4) fine rice genotypes, i.e. V1 (Kisan Basmati), V2 (Punjab Basmati), V3 (Chenab Basmati), and V4 (Super Basmati), were selected and exposed to four levels of NAA. The experiment was arranged under a completely randomised design (CRD) having three replications. There were 16 pots in one replication, which were replicated thrice. The seeds were water soaked for 24 hours and then sown in respective pots. After successful germination and seedling emergence, the plants were thinned to 3 plants/pot and were allowed to grow in normal conditions up to maturity. NAA was foliarly applied using a hand pump with different concentrations, i.e. N0 (control), N1 (20), N2 (30), N3 (40), and N4 (50) µmol/L, right before the flowering stage. Irrigation was applied by visual observation to keep the pots moist. No weeds were allowed to grow in the pots. Similarly, Furadan was also applied at the tillering stage to control the root or stem borer attack. Moreover, a fungicide was also applied at the time of flowering for the control of leaf blight. The irrigation was stopped one week before harvesting. The crop span was about 155 days (last week of June – last week of November) starting from sowing to harvesting. All the plants were harvested from each pot, tagged, and placed in the open air in sunshine for one week. Data regarding yield and quality traits were observed afterwards. Climate data were recorded for the growth period of the whole summer season (2017) i.e. June–Nov. It was revealed that June and July were recorded as the hottest months, while October and November were found as the coolest months. The maximum rainfall was recorded in the month of June and compared with others (Figure 1).
Yield parameters
The pots were harvested and tagged for recording the data of different yield parameters. All the samples were weighed and noted for biological yield (BY) per pot, which were converted to BY per hectare numerically. Similarly, 1,000 grains were counted from each treatment and weighed. Likewise, all the grains were separated manually and weighed for each pot or treatment, which was further converted to grain yield per hectare etc. The harvest index (%) was calculated from the following formula.
Quality parameters
Regarding kernel quality, normal kernels (filled grains) were separated from sterile kernels (un-filled) of each treatment and their percentage was determined. These parameters were measured manually using a light lamp and noted. For the determination of amylose contents, the method described by Juliano (1971) was used. Using a spectrophotometer, the intensity of blue colour was studied at 620 nm wavelength. Protein contents were determined with the Bradford method (1976). Grain length, breadth, and thickness (mm) were measured with a vernier caliper, whereas the elongation ratio (E/R ratio) was calculated by the following formula
Antioxidants
One week after the application of NAA, leaf samples were taken from each treatment and antioxidant activities were determined with the methods suggested by Wu et al. (2014), which are described as follows: Catalase (CAT) was determined with the procedure proposed by Hu et al. (2009). A 3.0 ml mixture was formed from 50 mM phosphate buffer, enzyme extract, and 15 mM H2O2. The activity of CAT was determined at 240 nm. Superoxidase dismutase (SOD) was determined as in Stewart and Bewley (1980). A 3.0 ml mixture was prepared using 50 mM phosphate buffer, 13 mM methionine, 0.1 µM EDTA, 75 µM NBT, enzyme extract and 2 µM riboflavin, which was further processed for 15 min and illuminated with 20 W florescent tubes, and reading was taken at 560 nm wavelength. Peroxidase (POD) activity was determined as in Shi et al. (2010). A 3.0 ml mixture was prepared by using 100 µl guaiacol (1.5%) v/v, 100 µl enzyme extract, 100 µl H2O2, 2 mM EDTA, and 2.7 ml potassium sulphate buffer. The mixture then underwent through processing and absorbance was read at 470 nm.
Auxin contents were determined using the method described by Hu et al. (2011). To this end, 0.5 g of fresh plant tissue was taken and homogenised in 50 ml of methanol/water (80/20, v/v), which contained 0.01 butylated hydroxytoluene (BHT), and blended in a high speed blender. After that, the solution was placed in −20 °C in a refrigerator for 1 day then centrifuged to collect the supernatant. The samples were analysed for Auxin contents using high-performance liquid chromatography (HPLC).
Statistical analysis
Statistical analysis of compiled data was done using Fisher’s analysis of variance in Statistix software, and significance of treatments was analysed by using the least significant difference (LSD) test at 5% probability (Steel et al., 1997).
. Results
Grain yield and quality
The yield parameters showed that Punjab Basmati (V2) and Kisan Basmati (V1) produced heavier grains (57%) under 40 µmol/L (N3), thereby enhancing the grain yield, and a 15.42% higher yield was recorded, compared with the control treatment. Similarly, V2N3 had a higher biological yield (14.77 t ha−1) and the harvest index (36.42%), which was almost 9.73% and 4.59% higher than in the control treatment, i.e. Super Basmati without NAA application (V4N0). The treatments without NAA resulted in the lowest performance in all the cultivars, with the lowest values observed in V4N0 (Table 1).
Table 1
Effect of naphthalene acetic acid (NAA) on yield parameters of various fine rice genotypes.
In terms of grain quality, the plants in the V2N3 treatment produced 80.64% of normal kernels, i.e. about 24% more than in the control treatment (V4N0). Similarly, V2N3 provided 53% less sterile kernels, compared to the control treatment (V4N0). As far as protein contents are concerned, both V2N3 and V1N3 expressed higher protein contents (11.85% and 11.74%, respectively), but the lowest protein contents were observed in V4N0 (10.43%). Likewise, the application of NAA decreased the amylose contents to increase the quality of grains. The V1N3 treatment combination contributed to production of 23.67% of amylose, which proved to be more reliable in decreasing the amylose level because it produced almost 15% less amylose contents in grains, compared to the control treatment (V4N0) (Table 2).
Table 2
Effect of naphthalene acetic acid (NAA) on quality parameters of various fine rice genotypes.
Grain cooking quality
Cooking quality is the major concern for fine rice. The results revealed that the NAA application improved the grain quality after cooking as well. The V1N3 treatment combination (Kisan Basmati with 40 µmol/L NAA) contributed to statistically higher grain cooking quality parameters, including grain length (7.84 mm), grain breadth (1.60 mm), grain elongation ratio (1.93), and grain thickness (1.38 mm), which were almost 4.5%, 28.12%, 27%, and 31% higher, respectively, than in the control treatment (V4N0). In turn, the lowest values of grain cooking quality parameters (7.50 mm, 1.15 mm, 1.40 mm, 0.95 mm of grain length, grain breadth, elongation ratio, and grain thickness, respectively) were observed in the control treatment (V4N0) (Table 3).
Table 3
Effect of naphthalene acetic acid (NAA) on grain quality of various fine rice genotypes after cooking.
Antioxidant activities and auxin contents
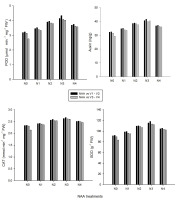
The V2N3 treatment combination (Punjab Basmati with 40 µmol/L NAA) exhibited the highest enzymatic activities, including Superoxidase dismutase (118.34 unit g−1 FW), peroxide (4.04 µmol min−1 mg−1 FW), and catalase (2.76 mmol min−1 mg−1 FW), which were 29%, 50%, and 22% higher than in the control treatment, i.e. Super Basmati without NAA application (V4N0), respectively. On the other hand, the control treatment (V4N0) displayed the lowest enzymatic activity (SOD 83.26 unit g−1 FW, POD 2.00 µmol min−1 mg−1 FW, CAT 2.14 mmol min−1 mg−1 FW). In contrast, the V2N3 treatment combination exhibited the maximum auxin content (26.06 ng/g), which was about 44% higher than in the control treatment (V4N0), as the control treatment had the lowest auxin contents (13.42 ng/g) during the study (Figure 2).
. Discussion
The data clearly indicates that the application of naphthalene acetic acid at the panicle stage proved beneficial in enhancing the growth, yield, and quality traits of the fine rice genotypes. Among the fine rice genotypes, V2 (Punjab basmati) produced higher results in various studied parameters. Vigorous growth was observed in the pots where NAA was applied, whereas decreased growth was noted in the pots without NAA application. This might be due to the improved level of auxin in the rice plants, which mitigated the heat or soil moisture stress and allowed the plants to grow normally, as auxin is an excellent growth regulator compound (Abou El-ghit, 2015; Li et al., 2019). Similarly, an optimum level of auxin in the plant enhances the rooting mechanism and improves the nutrient uptake (Kurpea & Smalle, 2020), potentially leading to improved crop productivity. Moreover, it has also been reported that the application of naphthalene acetic acid regulates plant growth, improves cell division, and development of associated organs (Basuchaudhuri, 2016; Hussain et al., 2021).
Abiotic stresses induce the production of ROS (reactive oxygen species), which suppress plant growth (Chauhan et al., 2022; Rehman et al., 2022). Plants have a mechanism to produce antioxidant enzymes, which improve homeostasis ROS and stress tolerance (Laxa et al., 2019). Abiotic stress causes many disabilities within plants, while a strong antioxidant system of plant acts as a scavenger of ROS (Kaya et al., 2020; Meisrimler et al., 2014). The current experiment revealed that the rice plants faced stress in the control or at a low dose application of NAA, resulting in reduced crop performance. Conversely, a higher dose of NAA improved plant stress tolerance to maintain the normal growth and reproductive process, ultimately leading to better crop yield. The results exhibited that antioxidant activities, including SOD, POD, and CAT, were enhanced by the application of naphthalene acetic acid (40 µmol L−1), potentially mitigating the effects of aerobic conditions or elevated temperatures. Earlier reports also revealed that the application of NAA had beneficial effects on increasing crop yield and antioxidants (Al-Duraid et al., 2019; Ullah et al., 2021). Similarly, plants treated with NAA (40 µmol L−1) also enhanced auxin contents, contributing to increased tolerance to withstand in unfavourable conditions, as auxin regulates many physiological functions in plants (Basuchaudhuri, 2016; Hu et al., 2011).
The crop growth was summed up in the grain yield; hence, the optimum growth ultimately had better results. The experiment results revealed optimised growth under the highest dose of NAA application, i.e. N3 (NAA @ 40 µmol L−1), as it improved the contents of auxin and antioxidants that scavenges ROS produced in a stressed environment. Naphthalene acetic acid N2 (NAA @ 20 µmol L−1) was slightly more prominent after N3, but the other doses did not give considerable results. The application of naphthalene acetic acid provides better growth, which makes plant gain additional total dry biomass and enhance yield traits (Alam et al., 2002; Bakhsh et al., 2011).
Grain quality is a major concern while growing fine rice, as its export and local consumption largely depend on this parameter. The results revealed that the crop grown under the NAA application improved grain quality, especially at its highest level of application. This may be attributed to the optimum growth of the rice crop, wherein the plants produced better dry matter, which may have been transferred towards grain for its optimum size. Similarly, the crop also completed the reproductive phase in normal conditions, as NAA boosted the auxin contents, improved defensive mechanisms, and reduced pollen sterility. It was also reported that auxin improves plant growth and panicle development in both normal and stress conditions (Abou El-ghit, 2015; Li et al., 2019). Due to this fact, the crop grown under NAA had reduced grain sterility and enhanced normal kernels. Moreover, this treatment also optimised other quality parameters like amylose and protein contents, which are a result of a better growing environment. The naphthalene acetic acid foliar application maximises protein production (Jahan & Adam, 2014).
Grain cooking quality traits are generally controlled by the genetic makeup of genotypes; however, a significant trend was observed with NAA spraying. The NAA dose of 40 µmol L−1 contributed to a better length, breadth, thickness, and elongation ratio of the fine rice genotype grains, whereas reduced values were observed in the control treatments. This may be attributed to the application of naphthalene acetic acid, as it promotes better plant growth and increased cell division, which may result in better yield and grain quality (Basuchaudhuri, 2016).
. Conclusion
The rice crop exhibited improvements in growth, yield, and quality parameters under the NAA application, with maximum results observed in the V2N3 (Punjab Basmati with 40 µmol/L NAA) combination. These increments may be due to the combined effect of an improved physiological mechanism in the rice plants, as evidenced by the auxin level and photosynthetic efficiency. The NAA application also improved the defence mechanism by enhancing antioxidant enzymes. More importantly, it also improved the rice grain quality at harvest and after cooking, which may further enhance the international trade. The results also revealed that the auxin application improved the pollen development and reduced the negative impact of elevated temperature on fertility and grain quality.