. Introduction
Rice (Oryza sativa L.; Os) is one of the most important economic crops serving as a primary food source for over half of the global population (Duong et al., 2023; Taratima et al., 2023). Thailand stands out as one of the world’s leading rice producers and exporters, with a production of 32.98 million tons in 2021 and 34.32 million tons in 2022 (FAOSTAT, 2021). This notable achievement is further enhanced by the country’s diverse array of rice germplasm. The most well-known Thai rice cultivars include fragrant rice (Khao Dawk Mali 105; ‘KDML105’), non-fragrant rice (Phitsanulok 2; ‘PSL2’), and glutinous rice (‘Niaw Ubon’). Among these, the Thai-indica rice cultivar ‘PSL2’ is extensively grown in Phitsanulok province, Thailand. The rice ‘PSL2’ was improved through a three-way cross between the ‘CNTLR81122-PSL-37-2-1’ / ‘SPRLR81041-195-2-1’ // ‘IR56’ lines by the Phitsanulok Rice Research Center during 1990–2000. It exhibits favorable agricultural traits, such as high yield productivity, grain quality, and resistance to brown planthopper and green rice leafhopper (Phitsanulok Rice Research Center, Thailand). However, one disadvantage of the ‘PSL2’ cultivar is its susceptibility to bacterial blight (BB) disease caused by the bacterium Xanthomonas oryzae pv. oryzae (Xoo) (Phitsanulok Rice Research Center, Thailand), leading to significant yield losses ranging from 30% to 60% (Yasmin et al., 2017). Currently, there are no effective methods for preventing the spread of bacterial Xoo, but most management strategies involve a combination of cultural, chemical, and genetic approaches (Khoa et al., 2017). One effective approach to decrease the severity of BB disease symptoms is the generation of an introgression line of resistance (R) genes to Xoo in the rice cultivar ‘PSL2’.
Of these R genes, the dominant Xa21 gene encodes a receptor-like protein kinase (RLK) and confers broad-spectrum resistance against Asian Xoo strains, triggering Xa21-mediated immunity (Peng et al., 2015). The RLK protein recognizes a specific Rax protein secreted by Xoo during rice infection (Liu et al., 2019). The formation of the RLK-Rax complex triggers a cascade of signaling events within the plant cell, leading to the activation of Xa21-mediated immune response pathways and defense-related proteins, including hypersensitive response (HR) programmed cell death at the site of infection (Radojičić et al., 2018). Thus, to enhance their resistance to BB disease, the Xa21 gene has been successfully introgressed into many susceptible rice cultivars, such as ‘RD47’ (Suachaowna et al., 2017), ‘CB174R’ (Govintharaj et al., 2021), and ‘Ciherang’ (Biswas et al., 2021) through marker-assisted selection in backcross breeding. For these reasons, introgression lines of ‘PSL2-Xa21’ in the BC4F5 generations were produced from a ‘PSL2’ × ‘IRBB21’ cross, followed by backcrossing to ‘PSL2’ for four generations, and then selfing for five generations during 2018–2021. In each generation, two gene-specific markers, pTA248 (tightly linked to the Xa21 gene) and Xa21, were used to identify the homozygous dominant genotype (Xa21/Xa21) in ‘PSL2-Xa21’.
Previous studies have reported that the Xa21 gene can also trigger a systemic acquired resistance (SAR) response in rice, allowing the rice to increase a long-lasting defense response throughout the entire plant. Additionally, salicylic acid (SA) plays a crucial role in plant defense against various pathogens. For example, SA accumulation triggers a series of responses, such as the activation of defense-related genes (Liang et al., 2022), restricts the growth of pathogenic Xoo, and reduces the BB disease severity in the Thai rice cultivar KDML105 (Thanh et al., 2017). The endogenous SA acts as a signal transducer (SA receptor) to subsequently induce the expression of genes related to the defense protein pathway, such as xa5 (Bimolata et al., 2015), OsNPR1 and OsTGA (Jiang et al., 2020), and OsPR1 (Schwessinger & Ronald, 2012). Moreover, SA enables the promotion of expression of genes OsWRKY45 and OsNAC4 related to the cell death pathway (Duan et al., 2014).
Overall, the combined interactions of the Xa21 gene and SA application might contribute to enhanced BB resistance, reduce Xoo proliferation, and limit Xoo spread during bacterial Xoo invasion. Thus, the interaction of the SA application and the Xa21 gene should be studied in response to BB disease symptoms in the introgression rice line ‘PSL2-Xa21’ in the BC4F5 generation.
. Materials and methods
Rice materials and plant genotyping
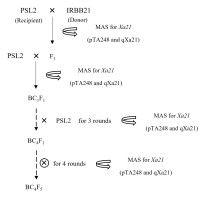
The rice introgression lines of ‘PSL2-Xa21’ in the BC4F5 generation were produced by marker-assisted selection (MAS) in a backcross breeding approach at the Faculty of Agriculture, Natural Resources and Environment, Naresuan University, Thailand. This process took place in greenhouse conditions with natural ventilation and sunlight, where the temperature ranged from 28.4 °C to 35.2 °C during the day and from 27.6 °C to 32.9 °C at night, with natural humidity levels between 63.5% and 92.9%. Briefly, the favorable Xa21 gene from rice cultivar ‘IRBB21’ (as the donor parent) was introgressed into ‘PSL2’ (as the recipient parent) to produce F1 seeds with the crossing method. These F1 plants were backcrossed to four ‘PSL2’ generations (BC4F1) to increase the genetic background of ‘PSL2’. Subsequently, the BC4F1 plants were self-pollinated for four generations to generate BC4F5 progenies to increase homozygosity (Figure 1). In each generation, the presence of the Xa21 locus was identified using a direct PCR assay with two specific MAS sets.
Figure 1
Schematic flowchart illustrating the introgression of the bacterial blight (BB) resistance gene, Xa21, from rice cultivar ‘IRBB21’ into ‘PSL2’ through the marker-assisted backcross (MAS) breeding approach. The two MAS markers used are pTA248 and qXa21. In each generation, eight introgression lines carrying the Xa21 gene are selected for further use.
The first marker, pTA248, was a co-dominance marker closely linked to the dominant Xa21 allele, which can amplify two different PCR products corresponding to 730 bp for the null-Xa21 allele (or recessive Xa21 allele) and 925 bp for the Xa21 allele (Yan-chang et al., 2004). The second marker, qXa21, was a dominance marker designed to specifically amplify a 114-bp DNA fragment of the published Xa21 sequence (GenBank no. U72723).
The base sequences of both markers are listed in Table 1. The selected plants ‘PSL2-Xa21-BC4F5’ were naturally grown and evaluated with similar and better agronomic traits than the recipient parent ‘PSL2’ in field conditions at Phitsanulok Rice Research Center, Thailand.
Table 1
Lists of primers and sequences used in this study.
| Primer name | Primer sequence (5′-3′) | Amplicon size (bp) | Reference |
|---|---|---|---|
| pTA248-F | 5′-AGACGCG GAAGGGTGGTTCCCGGA-3′ | 925 (Xa21), 730 (xa21) | Yan-chang et al. (2004) |
| pTA248-R | 5′-AGACGCGGTAATCGAAAGATGAAA-3′ | ||
| qXa21-F | 5′-CAGAGTATGGCGTTGGGCT-3′ | 114 | Promma et al. (2016) |
| qXa21-R | 5′-CGGGTCTGAATGTACTGTCA-3′ | ||
| Edf-F | 5′-TCCGAACCAGCAGATCATCG-3′ | 158 | Sagun et al. (2020) |
| Edf-R | 5′-GCATGGTATCAAAAGACCCAGC-3′ | ||
| Xoo4009-F3 | 5′-GTTCACCCTGCCCTTCATTTCCGTCGTCATC-3′ | 302 | Buddhachat et al. (2021) |
| Xoo4009-B3 | 5′-CCTTTGAGATCGCATGCATGAAGAACCACCACA-3′ |
PCR amplification
The rice cultivars of parents (‘PSL2’ and ‘IRBB21’), ‘PSL2-Xa21-BC4F1’, and ‘PSL2-Xa21-BC4F5’ were assigned the genotype of Xa21/Xa21, Xa21/xa21, and xa21/xa21 using MAS with a direct PCR approach of Phire Plant Direct PCR Master Mix®; (Thermo Fisher Scientific, UK) according to the manufacturer’s protocol. Briefly, leaf samples (1 mm) were crushed in 20 µL of dilution buffer and incubated for 5 min for genomic DNA (gDNA) extraction. The PCR reaction (20 µL) was prepared from the mixture of 1 µL of gDNA template, 1X Phire Direct PCR master mix, and 0.5 µM of each primer. This reaction was performed for DNA amplification in the following PCR conditions: pre-denaturation at 95 °C for 5 min, then 35 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s, extension at 72 °C for 30 s, and final extension at 72 °C for 5 min. The amplified PCR products were detected by 1.2% agarose gel electrophoresis.
Exogenous SA-pretreated and artificially Xoo-infected rice assay
Fifty-five-day-old rice plants (specifically during the flourishing tillering stage) were subjected to experimental foliar spray (50 mL/plant) once a day for two consecutive days with different concentrations of SA (0, 1, and 2 mM) on the whole plant. On the next day of pretreatment, five mature top leaves per plant were infected with Xoo16PK002 in greenhouse conditions. A distilled water (H2O)-treated rice leaf was used as the experimental control. In this experiment, the Xoo16PK002 bacterium was isolated from paddy fields in Phitsanulok province of Thailand, and was identified using an assay developed by Buddhachat et al. (2021). Meanwhile, the Xoo16PK002 culture and inoculation were carried out using the leaf-clipping method (Ke et al., 2017). At 7 and 14 days after inoculation (DAI), lesion lengths of three randomly sampled plants were measured and scored. The scoring criteria were as follows: high resistance (R, lesion length 0–5 cm), moderate resistance (M, lesion length >5–10 cm), moderately susceptible (MS, lesion length >10–15 cm), and susceptible (S, lesion length >15 cm) according to the IRRI Standard Evaluation System (International Rice Research Institute [IRRI], 2013).
The optimal SA concentration was selected for the quantification of gene expression profiles. The leaf samples were collected from the area below the clipping lesion, approximately 2 cm in size, at specific time points of 0, 1, and 2 hours after inoculation (HAI) with Xoo16PK002. Upon collection, the samples were immediately immersed in liquid nitrogen and stored at −80 °C until further use for gene expression analysis.
Meanwhile, in the same experimental sample above, leaf samples (1 × 1 cm2) from the inoculated area below the clipping lesion (approximately 2 cm) of both exogenous SA- and H2O-pretreated rice plants were harvested at 3 DAI, following a modified method based on Thanh et al. (2017). The harvested sample was followed by surface sterilization with sterilized distilled water and 70% ethanol. Subsequently, the leaf sample was finely chopped into small pieces and placed into sterilized distilled water (100 µL). The mixture was then incubated at 95 °C for 10 min. The supernatant (2 µL) of crude plant extracts was directly used for Xoo quantification using the qPCR system with the Xoo4009-F/R marker pair (Table 1). The remaining supernatant was stored at −20 °C for further use.
The symptomatic response to BB disease was evaluated and photographed at 14 and 21 DAI by measuring the lesion lengths of three plants, and then scored according to the IRRI Standard Evaluation System (IRRI, 2013).
qPCR analysis
The expression profile of the Xa21 gene in the rice samples was determined using the qPCR assay. Total RNA was extracted from a leaf region below the clipping lesion (approximately 5 cm) using the FavorPrepTM Plant Total RNA Mini Kit (Favorgen, Taiwan) following the manufacturer’s instructions. The genomic DNA contamination in the total RNA sample was further eliminated with RNase–Free DNase I treatment (Thermo Scientific, USA). Subsequently, the quality and integrity of the total RNA were evaluated through gel electrophoresis, and the concentration was determined by measuring absorbance at A260 nm and A280 nm using a NanoDropTM Lite Spectrophotometer (Thermo Fisher Scientific, USA). The pure RNA sample was indicated by a value of the A260/A280 ratio ranging from 1.8 to 2.0. First-strand cDNA synthesis was performed using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Baltics, UAB, LT) according to the manufacture’s protocol. The qRT-PCR reaction was carried out using the Thermo Scientific Maxima SYBR Green qPCR Master Mix (Thermo Fisher Scientific, Baltics, UAB, LT) on the Eco48 Real-Time PCR system (EcoTM48, PCRmax Limited, UK). The thermal cycling conditions were as follows: an initial denaturation step at 95 °C for 10 min, followed by 35 cycles (95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s). A melting curve was realized by progressively heating the reaction mixture from 55 °C to 95 °C using 0.3 °C increments every 0.75 s to check the purity of the qRT-PCR product. The average threshold cycle (Ct) value was used to calculate the fold change of gene expression. The expression of the Xa21 gene was normalized to the expression of the Endothelial Differentiation Related Factor (edf) gene (Sagun et al., 2020). The 2–ΔΔCt method was applied for relative quantification (Livak & Schmittgen, 2001). All reactions were technically repeated three times with at least three biological replicates. The primer sequences used in the qPCR assay are listed in Table 1.
Moreover, the number of bacterial Xoo copies (CFU/mL) was quantified by the thermal cycle of qPCR on the Eco48 Real-Time PCR system (EcoTM48, PCRmax Limited, UK) in the following conditions: 1 cycle of pre-denaturation at 95 °C for 5 min, followed by 40 cycles (95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s), and final extension at 72 °C for 5 min. The Ct values obtained from the Xoo sample were used to calculate the bacterial numbers by comparing them with the standard curve. This curve was generated from a 10-fold serial dilution of the Xoo cell suspension (ranging from 25 to 2.5 × 108 CFU/mL) of known concentrations from the initial Xoo cell suspension at OD600 = 0.2 (equivalent to 2.5 × 108 CFU/mL).
Statistical analysis
The experiment was conducted using a complete randomized design (CRD), with three to five biological replicates. The results were expressed as the mean value ± standard error. Data analysis was performed using IBM SPSS Statistics version 19. Statistically significant differences among the treatment means were determined using Duncan’s new multiple range test (DMRT) at p value ≤ 0.05 or 0.01.
. Results
Marker-assisted selection of the Xa21 gene
To identify the Xa21 genotypes across the rice cultivars, the gDNA template extracted from nine introgression lines (‘PSL-Xa21-BC4F5’), ‘PSL-Xa21-BC4F1’, and two parental lines (‘PSL2’ and ‘IRBB21’) was used to amplify the PCR product with two functional pTA248 and qXa21 markers related to BB disease resistance. Based on the pTA248 co-dominant marker analysis, the homozygous dominant genotype (Xa21/Xa21) was found in the rice cultivar ‘IRBB21’ and all introgression lines ‘PSL-Xa21-BC4F5’, which amplified a single 925-bp PCR product. The introgression line ‘BC4F1’ plant exhibited both 730-bp and 925-bp PCR products corresponding to the heterozygous Xa21 genotype (Xa21/xa21). In turn, the ‘PSL2’ showed a single 730-bp PCR product, confirming the homozygous recessive genotype (xa21/xa21) (Figure 2A).
Figure 2
Plant genotyping analysis across 12 rice cultivars (‘PSL2’, ‘IRBB21’, ‘BC4F1’, and ‘BC4F5L1-L9’ plants) using the pTA248 (A) and qXa21 (B) markers. In each PCR reaction, the DNA template was derived from the rice cultivar ‘PSL2’ (lane 1 and 4); ‘IRBB21’ (lane 2 and 5); ‘BC4F1’ (lane 3); ‘BC4F5L1’–‘BC4F5L9’ (lane 6–14), listed in Table 2. Lane M represents the 100 bp DNA ladder.
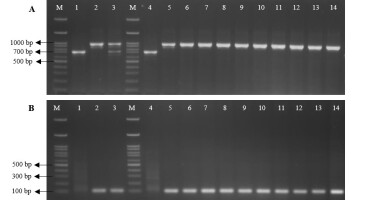
Similarly, the presence of the Xa21 genotypes among these introgression lines ‘PSL-Xa21’ lines was confirmed using the qXa21-F/R dominant marker. The result showed that all the BC4F5 lines exhibited only a single PCR product, corresponding to a 114-bp long product (Figure 2B), indicating that all the BC4F5 lines contained the homozygous Xa21 genotype (Xa21/Xa21) (Figure 2B). These findings provide strong evidence that all the ‘PSL-Xa21-BC4F5’ lines possess the homologous dominant alleles of the Xa21 resistance gene.
Agronomic performance of introgression lines
The selected introgression lines carrying the homozygous Xa21 genotype underwent a comprehensive evaluation of their agronomic performance in field conditions. The results revealed that the nine introgression lines (without Xoo16PK002 infection) exhibited similar or better agronomic performances (days to flowering, plant height, panicle number and length, filled grains per panicle, 100-grain weight, and total grain weight per plant) than the recipient parent ‘PSL2’ (Table 2). These findings indicate the successful incorporation of the Xa21 gene without compromising the overall agronomic performance.
Table 2
Morpho-agronomic performances of nine-introgression lines ‘PSL2-Xa21-BC4F5’ relative to their donor and recipient parents.
[i] All the studied rice cultivars were grown in separate pots in natural greenhouse conditions. Data are expressed as the mean value ± standard error of at least 3 biological replicates. Different letters within the same column indicate statistically significant differences analyzed by DMRT at p ≤ 0.01. * R, high resistance; S, susceptibility.
Furthermore, all the tested introgression lines also showed a high level of BB resistance, as evidenced by the lesion lengths ranging from 4.80 ± 1.9 cm to 3.8 ± 0.33 cm at 21 days after the Xoo16PK002 inoculation, compared to the BB-resistant cultivar ‘IRBB21’ and the BB-susceptible cultivar ‘PSL2’ (Table 2, Figure 3). Among these, two introgression plants, BC4F5-L1 and BC4F5-L8, had the highest total grain weight per plant, measuring 89.01 ± 10.66 g and 90.04 ± 3.52 g, respectively. Based on these promising results, these two selected lines were further propagated through self-pollination to generate ‘BC4F6’ seeds and used for further experiments.
Effect of exogenous SA pretreatment on BB resistance
To optimize the exogenous SA concentration for BB resistance in rice, whole plants of ‘PSL2’ (at 55 days old) were pretreated with either SA (0.5, 1, and 2 mM) or H2O, and the following day, the plants were inoculated with the Xoo16PK002 isolate in greenhouse conditions. Overall, the results showed that the higher concentrations of SA trended towards reducing the lesion length in BB disease. Among these, the SA pretreatment with 2 mM significantly reduced the severity of BB in the rice ‘PSL2’ at 7 DAI, with a lesion length on the leaf of 6.35 ± 0.11 cm, compared to the other treatments (Figure 4). Similarly, at 14 DAI, the SA pretreatment with both 1 or 2 mM resulted in a more significant reduction of BB lesion length (15.41 ± 0.24 and 15.10 ± 0.31 cm, respectively) than the H2O pretreatment control (16.47 ± 0.39 cm) (Figure 4). These findings indicated that the exogenous SA pretreatment could enhance systemic BB resistance against Xoo16PK002 in the rice cultivar ‘PSL2’. Therefore, the role of the SA-mediated gene (such as Xa21) conferring BB disease resistance should be further studied.
Figure 4
Effect of different concentrations of the SA pretreatment on lesion length (A) and symptomatic (B) progression of BB disease in rice ‘PSL2’ at 7 and 14 DAI. Fifty-five-day-old rice plants (at the flourishing tillering stage) were pretreated twice with SA (1 and 2 mM) or H2O on consecutive days (on days 55 and 56 after plantation) and then subsequently inoculated with the Xoo16PK002 isolate on day 57 after plantation.

SA-mediated Xa21 expression in the introgression line ‘PSL2-Xa21’
To determine the influence of the SA pretreatment on the Xa21-gene expression in rice responding to Xoo, two-introgression lines (‘PSL2-Xa21-BC4F6-L1 and L8’) were experimentally subjected to SA (2 mM) or H2O pretreatment, and then subsequently infected with the Xoo16PK002 isolate in greenhouse conditions. Leaf samples were collected at different time points (0, 1, and 2 hours after inoculation; HAI) to quantify gene expression with the qRT-PCR assay. In the SA-pretreated rice cultivars ‘IRBB21’ and ‘PSL2-Xa21-BC4F6-L1 and L8’, the Xa21 gene showed slightly higher expression of Xoo16PK002 at 0 HAI, compared to the mock pretreatment (Figure 5A). After that, Xa21 trended to be slightly down-regulated at 1 and 2 HAI of Xoo16PK002, compared to 0 HAI of Xoo16PK002 under the SA pretreatment (Figure 5A). These results indicated that the SA-pretreated rice might stimulate the Xa21 gene expression before Xoo16PK002 infected the rice. In contrast, under the H2O pretreatment, Xa21 showed higher expression at 2 HAI of Xoo16PK002 in all the studied rice cultivars, compared to 0 HAI of Xoo16PK002 (Figure 5A). However, no Xa21 gene expression was detected in the rice ‘PSL2’ carrying a homozygous recessive genotype (xa21/xa21) (Figure 5A). Summarized results indicated that Xa21 gene expression could be induced by either exogenously SA-pretreated or post Xoo-infected rice.
Figure 5
Influence of the SA pretreatment (2 mM) on Xa21 expression and BB disease resistance in rice introgression lines. (A) Xa21 gene expression in the conditions of SA (2 mM) or H2O pretreatment and Xoo16PK002-inoculated rice cultivars (‘IRBB21’, ‘PSL2’, and introgression lines) at 0, 1, and 2 HAI; (B) Bacterial populations of Xoo16PK002 in rice plants. The number of bacteria in 1 cm leaf sections from the cut sites was measured at 3 DAI; (C–D) Lesion length on leaves of rice cultivars (‘IRBB21’, ‘PSL2’, and two-introgression lines) infected with the Xoo16PK002 isolate at 14 (C) and 21 DAI (D). Values are the mean ± SE of three independent biological replicates. Letters denote significant differences based on analyses of plants treated with SA and H2O using the DMRT (p ≤ 0.05).
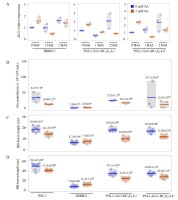
To assess Xoo16PK002 multiplication in the rice, the inoculated leaves from both the SA and H2O pretreatments were collected at 3 DAI, and Xoo16PK002 copies were quantified with the qPCR assay. In overall results, the SA-pretreated rice cultivars (‘PSL2’ and ‘PSL2-Xa21-BC4F6-L1 and L8’) had significantly fewer Xoo copies (ranging from 12.8 × 104 to 18.1 × 104 CFU/mL) than the H2O-pretreated control (ranging from 25.8 × 104 to 35.7 × 104 CFU/mL) (Figure 5B). Of these, the SA-pretreated rice cultivar ‘PSL2’ exhibited a significantly lower number of Xoo copies (14.0 × 104 CFU/mL) than in the H2O pretreatment variant (34.7 × 104 CFU/mL), resulting in an approximately 2.4-fold reduction in Xoo16PK002 proliferation (Figure 5B). However, the rice cultivar ‘IRBB21’ in both the SA and H2O pretreatments showed non-significant differences in the number of Xoo copies, i.e. 2.8 × 104 and 2.0 × 104 CFU/mL, respectively (Figure 5B). These results indicated that exogenous SA application in rice cultivars could be a potential strategy against BB disease severity through suppression of Xoo proliferation in rice plants.
To evaluate the effect of SA on reducing BB symptoms after Xoo16PK002 inoculation in rice, the results revealed that the SA pretreatment (2 mM) significantly reduced the BB severity in the rice ‘PSL2’, with lesion length on the leaves of 14.1 cm and 41.4 cm at 14 DAI (Figure 5C) and 21 DAI (Figure 5D), respectively, compared to the mock treatment (18.6 cm at 14 DAI and 50.4 cm at 21 DAI). Additionally, the two-introgression lines ‘PSL2-Xa21-BC4F6-L1 and L8’ exhibited moderate BB resistance, with lesion length on the leaves ranging from 10.0 to 10.9 cm at 14 DAI, compared to the H2O-pretreated control (16.8–18.0 cm) (Figure 5C). Similarly, at 21 DAI, these lines also exhibited moderate BB resistance with lesion lengths ranging from 24.6 cm to 28.0 cm, compared to the H2O mock pretreatment (34.9–35.1 cm) (Figure 5D). In contrast, both the SA-pretreated rice and H2O-pretreated rice cultivar ‘IRBB21’ showed slight differences in the lesion length on the leaves at 14 and 21 DAI (Figure 5C–D). These findings indicated that the SA treatment could significantly reduce symptomatic BB severity in the susceptible rice cultivar ‘PSL2’ and the introgression lines ‘PSL2-Xa21’, but not in the rice cultivar ‘IRBB21’.
In summary, the SA-pretreated rice of two introgression lines ‘PSL2-Xa21’ exhibited reduction in symptomatic BB disease and Xoo16PK002 multiplication. However, these lines showed moderate BB resistance to Xoo16PK002 than the parental cultivars ‘IRBB21’ and ‘PSL2’.
. Discussion
The utilization of backcross breeding, along with marker-assisted selection (MAS), is a valuable approach for transferring desired traits from a donor parent into the favored genetic background of a recurrent (or recipient) parent. Additionally, the MAS has proven to be an essential tool in breeding programs, as it enables efficient and cost-effective selection of individual plants carrying desirable genes from large populations (Govintharaj et al., 2018). Based on the mode of gene action and inheritance, the co-dominant marker is more effective in discriminating between homozygous and heterozygous genotypes of individuals than the dominant marker (Collard et al., 2005). In this study, the pTA248 co-dominant marker clearly discriminated three different genotypes (Xa21/Xa21; Xa21/xa21; and xa21/xa21) among the studied rice lines. The pTA248 marker enabled the amplification of a single PCR product with clearly different sizes between the dominant Xa21 allele and the recessive xa21 allele, corresponding to 925-bp and 730-bp length, respectively. In agreement with previous findings, the Xa21-gene-linked marker (pTA248) was successfully utilized as MAS to identify the Xa21 gene target in many introgression rice cultivars, such as ‘indica RD47’ (Suachaowna et al., 2017), ‘CSR3’ (Baliyan et al., 2018), ‘Super Basmati’ (Sabar et al., 2019), ‘Putra-1’ (Chukwu et al., 2019), and ‘Ciherang’ (Biswas et al., 2021). This finding suggests that the pTA248 co-dominant marker has high accuracy and greatly improved selection efficiency to identify individual plants carrying the desirable gene in the early generations of rice breeding programs.
From previous knowledge, SA is a crucial natural signaling molecule that plays a key role in triggering the activation of the SAR system (Noshi et al., 2016). Additionally, it also activates the SA-dependent signaling pathway by regulating disease resistance mechanisms, such as hypersensitive responses (HR) and defense genes such as xa5, OsNPR1, and OsWRKY45 (Jiang et al., 2020), and inhibits pathogen growth (Gális et al., 2004). As shown in Figure 4, the exogenous SA application (at 1 and 2 mM) to the susceptible rice cultivar ‘PSL2’ significantly suppressed BB-disease progression by exhibiting the shortest lesion length at 7 and 14 DAI of Xoo16PK002, compared to the mock-pretreated control. A similar finding was reported by Shasmita et al. (2019), who observed that exogenous SA treatment (at 2 mM) in the rice cultivar ‘Taichung Native 1 (TN1)’ provided the most significant reduction in BB disease severity caused by Xoo pathogens. More recently, Thepbandit et al. (2021) reported that an optimal concentration range of SA (1–3 mM) conferred the highest efficiency in enhancing BB resistance in the rice cultivar ‘KDM105’. This phenomenon suggested that SA functions as an elicitor to induce BB resistance mechanisms. For instance, the SA inducer may be associated with SAR mechanisms, particularly in transmitting defense signals (Suharti & Leana, 2021). Additionally, the SA elicitor may activate HR, leading to cell death at the site of infection (Radojičić et al., 2018), as well as morphological changes, such as increased leaf epidermis thickness and lignin formation in plant tissues (Kavulych et al., 2019).
In this study, the results showed that the SA-pretreated rice cultivar ‘IRBB21’ and the backcrossed rice lines ‘PHS2-Xa21-BC4F6’ were capable of inducing an earlier and higher expression level of the Xa21 gene before Xoo16PK002 infection (at 0 HAI), compared to the mock pretreatment. However, there is limited evidence to support the hypothesis that exogenous SA application can directly induce Xa21 gene expression in rice. Only one study has shown that SA signaling positively responded to activate xa5-mediated immunity to BB disease in of rice cultivar ‘IRBB5’, whereas its endogenous SA levels were also especially induced after infection by the Xoo strain, PXO86 (Jiang et al., 2020). In contrast, in the H2O-pretreatment conditions, the Xa21 gene expression was significantly upregulated at a later time point, specifically 2 HAI of Xoo16PK002 infection in the rice cultivars ‘IRBB21’ and the introgression lines ‘PSL2-Xa21-BC4F6’. This finding was consistent with previous observations that Xa21 gene expression was up-regulated at 1–2 HAI in rice ‘IRBB21’ and ‘RD47-Xa21’ (Sagun et al., 2019). Similarly, Xa21 gene expression has also been shown to confer a higher level of BB resistance with reduced lesion lengths post Xoo-inoculated rice harboring Xa21-resistance gene lines, compared to susceptible lines (Gao et al., 2018; Hu et al., 2015). This might be explained by the fact that the Xa21-mediated immune response is dependent on the presence of bacterial Rax proteins, translated from the Rax operon, which are required for activating Xa21 gene expression (Pruitt et al., 2015) and promoting BB resistance in rice (Gao et al., 2018).
In this study, the SA-pretreated rice cultivars ‘PSL2’ and ‘PSL2-Xa21’ were found to effectively suppress the proliferation and growth of Xoo16PK002 cells at 3 DAI. Additionally, it was observed that the SA pretreatment led to a reduction in the severity of BB disease caused by Xoo16PK002 at 7 and 14 DAI. One explanation was that the SA elicitor could potentially trigger a positive immune response against Xoo infection (Jiang et al., 2020). This response could lead to a partial restriction of bacterial Xoo proliferation and induce an HR to prevent bacterial spread within rice plants (Radojičić et al., 2018). Moreover, the SA elicitor might be involved in the induction of various immune regulators contributing to plant defense against Xoo infection. In agreement with previous findings, the increased SA concentration could trigger rapidly localized cell death (known as the HR) at the specific site of pathogen invasion. This mechanism has proven effective in limiting the symptomatic progression of BB disease (Balint-Kurti, 2019). High levels of SA in rice cultivars (‘KDML105’, ‘IRBB5’ or ‘IR24’) have been shown to strongly reduce Xoo multiplication (Thanh et al., 2017; Zhang et al., 2013) at 8 hours post infection (Jiang et al., 2020). Additionally, the regulator of SA signaling (OsNPR1 proteins) enhances the plant defense response by repressing growth and reducing Xoo virulence by down-regulating the expression of multiple virulence genes and genes involved in energy metabolism, biosynthesis, and nutrient transportation (Dai et al., 2023). Taken together, these findings indicated that the SA pretreatment with 2 mM in the rice cultivar ‘PSL2-Xa21’ induces up-regulation of the Xa21 gene and can significantly decrease bacterial Xoo proliferation against the symptomatic BB disease in rice.
. Conclusion
This study aimed to determine the effect of SA application on BB resistance in rice cultivars (‘PSL2’, ‘IRBB21’, and backcrossed lines ‘PSL2-Xa21-BC4F6’) after Xoo16PK002 inoculation, compared to the mock treatment control. The results showed that the SA-pretreatment (2 mM) significantly upregulated the Xa21 gene expression in the rice cultivars ‘IRBB21’ and ‘PSL2-Xa21-BC4F6’ before Xoo16PK002 infection. Furthermore, the SA-pretreatment of the rice led to the suppression of Xoo16PK002 proliferation and increased BB disease resistance in the rice cultivars ‘IRBB21’ and ‘PSL2-Xa21-BC4F6’ after Xoo16PK002 inoculation. However, these introgression lines still showed moderate BB resistance to Xoo16PK002, compared to the rice cultivar ‘IRBB21’. These findings could indicate that BB disease resistance in rice might not only depend on the exogenous SA treatment and the Xa21 resistance gene but could also be associated with other signaling mechanisms. Thus, the combined functions of SA and Xa21 should be further studied to gain valuable insights into BB resistance mechanism against Xoo invasion in rice.



