. Introduction
Orchids are commercially important plants because of their exotic values, such as the variety in colors, sizes, shapes, fragrances, and long vase life of their flowers. A number of orchids have medicinal importance (Park et al., 2018). These plants are cultivated as cut flowers and pot plants around the world (Park et al., 2018). Red Helleborine Cephalanthera rubra (Orchidaceae), one of the rarest orchids, is a broad-leaf herb orchid found in Europe, North Africa, southwest Asia, and in Iran. The geographical distribution of this species covers Europe-Siberia, the Mediterranean region, and Iranian Turani. The phenology of this plant is a geophyte containing rhizomes. Its shoots grow from a creeping rhizome and have between 2 and 8 lanceolate leaves. Each shoot may carry up to 20 flowers, which may be pink to red or rarely white (Brickell & Zuk, 1997). The importance of this species is that it is ornamental. Its other importance, including its medical and pharmaceutical role, is not fully known.
Orchids are facing extinction in the wild due to many factors, especially human activities. There is an increased demand in orchid cut flowers and potted plants because of their exotic beauty and long shelf life (Das et al., 2021). Preservation of plant biodiversity is necessary for plant breeding, genetic engineering programs, sustainable development of valuable genotypes and cell lines, and a source for use in some industries (Kulus, 2020).
Cryopreservation is the only safe, reliable, and cost-efficient technique for long-term storage of biological materials (i.e., seeds, shoot tips, dormant buds, zygotic, and somatic embryos, cell suspensions, callus tissues, and pollens) or germplasms at cryogenic temperatures (LN; −196 °C or in its vapor phase, LNV; −150 °C to −196 °C) mainly without losing viability and altering the genetic makeup. In these conditions, biological materials are theoretically preserved indefinitely, because metabolic processes and cell division are arrested. Cold preservation and cryopreservation facilitates effective storage of plant genetic resources with high fidelity, minimum risk of genetic erosion, and low cost with minimal requirements of space and equipment for long term (Benelli et al., 2021; Bettoni et al., 2021; Downey et al., 2021; Valdés et al., 2021; Zhang et al., 2023).
Germplasms can be encapsulated in calcium alginate beads, such as in the encapsulation-dehydration technique, to improve the effectiveness of cold storage and cryopreservation and enhance explant recovery without genetic erosion. The selection of an optimal cryopreservation technique is often species dependent (Kulus, 2020). Most plant cells and tissues contain high amounts of intracellular water that should be artificially dehydrated before exposure to LN. This may be done either physically and/or chemically mainly by air-desiccation and high concentrations of cryoprotectants, especially sucrose, respectively (Kaviani & Kulus, 2022). Therefore, cryopreservation is usually lethal for biological materials exposed to ultra-low temperatures of LN without any pretreatment because of intracellular water freezing (Hirano et al., 2009).
A very small percentage of orchid genera and species have been evaluated for cold storage and cryopreservation conditions. It is very important to find effective protocols for cold protection and, especially cryogenic protection of endangered orchids with cheap and reliable methods and types of explants. In vitro storage, especially cold storage and cryopreservation techniques, can be employed for crops difficult or impossible to keep in seed banks for long-term conservation, including woody perennial plants, recalcitrant seed crops or crops without seeds, and clonally propagated crops where seeds are not true-to-type (O’Brien et al., 2021). Cold preservation or the slow-growth storage technique for medium-term conservation can be established by keeping plant germplasms at a low temperature (usually 4–15 °C). Some studies have been reported for cold storage of endangered ornamental plants, including orchids, with varying results (Manokari et al., 2021; Singh et al., 2015; Teixeira da Silva et al., 2014).
Cryopreservation techniques were reported for several orchids species, such as Dendrobium, Cymbidium, Bletilla, Phalaenopsis, and Vanda genera (Asa & Kaviani, 2020; Das et al., 2021; Imsomboon & Thammasiri, 2020; Kaviani & Kulus, 2022; Khoddamzadeh et al., 2011; Kulus & Zalewska, 2014; Mohanty et al., 2012; Pimda & Bunnag, 2010; Subramaniam et al., 2011; Surenciski et al., 2012; Vendrame et al., 2014). Most of these researchers used the encapsulation-dehydration technique as pretreatment for cryopreservation. The short-term cold storage technique by encapsulation-dehydration has been applied in a few orchids (Manokari et al., 2021).
The application of shoot tips as explants for cold storage and cryopreservation of orchids has been reported very little. The seed, protocorm, and protocorm-like body (PLB) are the most commonly used explants for cold storage and cryopreservation of orchids (Das et al., 2021; Imsomboon & Thammasiri, 2020; Kaviani, 2011; Kaviani & Kulus, 2022; Kulus & Zalewska, 2014; Popova & Kim, 2019; Wu et al., 2013). In orchids, successful cryopreservation using seeds, protocorms, and PLBs and through various techniques has been reported. The seeds of some orchid species have been demonstrated to lose viability immediately after desiccation (Wu et al., 2013). One of the most important advantages of using somatic tissues, such as shoot tips and apical meristems, for germplasm conservation is that specific plant genotypes are preserved, resulting in commercially valuable stocks. On the other hand, utilization of these explants has resulted in rapid propagation of many orchids. Cold storage and cryopreservation techniques for endangered orchids and used explants should be optimized (Das et al., 2021).
The decline and destruction of the natural habitat and indiscriminate collection for ornamental use have been decreasing orchid populations dramatically, particularly those in danger of extinction, and their genetic resources. Hence, it is inevitable to preserve the genetic resources of orchids in any possible way. Therefore, the present study, carried out for the first time on C. rubra (L.) Rich., aimed to evaluate the effect of different pretreatments, especially encapsulation-dehydration, on cold storage and cryopreservation of this rare orchid using shoot tip explants.
. Material and methods
Plant material and disinfection
Plant material (Cephalanthera rubra (L.) Rich.) was collected from a jungle of Dodangeh, Sari city, Mazandaran province, the northern part of Iran. Cuttings were taken from the stem containing a shoot tip with two young leaves. Plant materials were transferred to a Plant Tissue Culture Lab in Amol city, Mazandaran province, the northern part of Iran. The geographical coordinates of Amol are as follows: latitude: 36°28′10″ N, longitude: 52°21′02″ E, and elevation above sea level: 96 m (314 ft). Shoot tips (10–15 mm in length) were used as explants. To eliminate surface contamination, the explants were washed carefully with a dishwashing liquid together with a drop of Tween-20 for 20 min. Next, the explants were placed under running tap water for half an hour. After preliminary washing, all the explants were surface-sterilized by full immersion in a 15% (v/v) sodium hypochlorite (NaOCl, commercial bleach) solution supplemented with 0.1% Tween-20 for 10 min. Then, the samples were disinfected with 0.1 mg l−1 (w/v) mercury chloride (HgCl2) for 10 min followed by washing in distilled water for 15 min. Finally, ethanol 70% was used for one min. All the disinfection steps were performed under a laminar air-flow cabinet and accompanied by periodic gentle agitation. The disinfected explants were then rinsed by agitating in sterile distilled water three times for a total of 15 min. Using sterile forceps, the shoot tips were transferred to autoclaved ashless WhatmanTM filter paper to dry briefly. To remove the parts of the explants damaged in the disinfection treatments, the margins of the explants were cut off.
Encapsulation-dehydration procedure
All cryoprotectant solutions used in different processes of the protocols were prepared in liquid Murashige and Skoog (1962) (MS) medium (salts and vitamins, pH 5.7 ± 1.0) and sterilized by an autoclave. For encapsulation, 3% Na-alginate (w/v) and 100 mM calcium chloride (CaCl2⋅2H2O) prepared in MS basal medium were applied. The shoot tips with two young leaves were subjected to an encapsulation-dehydration cold preservation and cryopreservation procedure. The shoot tips were embedded in 3% Na-alginate in liquid MS medium without calcium salt for 2 h at room temperature. Then, alginate droplets containing the shoot tips were individually dropped into a MS medium with a 100 mM CaCl2⋅2H2O solution using sterile forceps and left for 2 h at room temperature with gentle agitation to form beads. Encapsulated shoot tips were utilized for cold preservation (slow-growth storage) and cryopreservation by encapsulation-dehydration. For physical dehydration (air-desiccation), encapsulated explants were placed in uncovered sterile Petri dishes (9 cm Ø) and kept for 2 h under a stream of aseptic air in a laminar air-flow cabinet. For chemical dehydration, 0.75 M sucrose was added in MS basal medium through capsule formation.
In vitro conservation by slow-growth storage
For in vitro conservation, shoot tips encapsulated in alginate beads with or without 0.75 M sucrose were transferred to Petri dishes, sealed with parafilm, and stored at 4 °C in the dark for 6 months. There were five explants in each of the four Petri dishes. In non-encapsulated pretreatments, shoot tips were put into liquid MS medium together with 0.75 M sucrose with or without air-desiccation for 2 h.
Cryopreservation by encapsulation-dehydration
For cryopreservation by encapsulation-dehydration, shoot tips encapsulated in alginate beads with or without 0.75 M sucrose and air-desiccation for 2 h were placed into pre-sterile 2 ml polypropylene cryogenic vials (Figure 1). The vials were sealed and quickly immersed in LN for at least 1 h. Different combinations of pretreatments (shown in Table 1) were used to reveal the impact of each step and optimize the encapsulation-dehydration procedure. In non-encapsulated pretreatments, explants were put into liquid MS medium with 0.75 M sucrose with or without air-desiccation for 2 h.
Figure 1
Shoot tips of Cephalanthera rubra (L.) Rich. as explants. (A) Non-encapsulated shoot tips; (B) encapsulated shoot tips; (C) shoot tips within cryovials ready for cryopreservation. Scale bars = 5 mm.
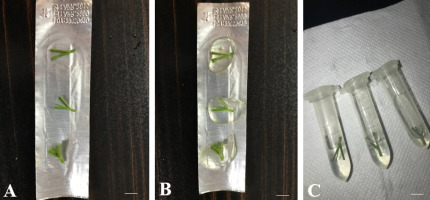
Table 1
Different pretreatments applied for shoot tips in experiments on cold (slow-growth) storage and cryopreservation by encapsulation-dehydration.
Effect of pretreatments
Different combinations of sucrose pretreatments, encapsulation, and air-desiccation were tested to reveal the impact of each step of the protocol on the survival of the shoot tips after cold storage and cryopreservation by encapsulation-dehydration (Table 1). For sucrose pretreatment, 0.75 M sucrose was added in MS basal medium. In non-encapsulated treatments, explants were put into liquid MS medium with 0.75 M sucrose prior to other manipulations.
Rewarming of cryopreserved explants
After at least one hour of storage of the shoot tips in LN for cryopreservation by encapsulation-dehydration, cryovials holding the shoot tips were removed from LN and rewarmed rapidly by immersing in 39 ± 1 °C water bath for 3 min.
Recovery and regeneration medium
The same regeneration medium and environmental conditions were used in cold storage and cryopreservation experiments. After 6-month storage of shoot tips under in vitro conservation by slow-growth storage or rewarming after cryopreservation, beads containing shoot tips were removed from the vials and inoculated in an actively growing state on recovery MS medium fortified with 3% sucrose, 0.7% agar, and 4 mg l−1 BA formulated earlier for shoot multiplication (data not shown). A variety of recovery media were investigated using some plant growth regulators (PGRs), including α-naphthalene acetic acid (NAA), 2,4-dichlorophenoxyacetic acid (2,4-D), and 6-benzyladenine (BA) at different concentrations (data not shown). The best results from the primary in vitro propagation were selected for the recovery medium. The pH was adjusted to 5.7 ± 1.0 using 0.1 N hydrochloric acid (HCl) or sodium hydroxide (NaOH) before adding agar and prior to autoclaving the medium for 20 min at 105 kPa and 121 °C. All cultures were maintained in a growth room at a temperature of 23 ± 2 °C with a relative humidity of 70 ± 5%, in standard growth conditions of the 16/8 h photoperiod regime provided by cool-white fluorescent lamps with a photosynthetic photon flux density (PPFD) of approximately 40 µmol m−2 s−1.
Determination of survival
For cold storage and cryopreservation, post-thaw survival rate data (percentage of shoot tips that maintained their green color and vigor but did not show visible growth) were examined weekly after rewarming and transfer to the regeneration medium. The survival capacity (recovery level or regrowth rate) in terms of emergence of leaves or roots from the beads was measured 60 days after rewarming. The total number of dissected shoot tips was considered 100%. In other words, the survival percentage was calculated as the number of beads germinated among the total countered number of beads. Completely brown or white shoot tips were denoted as dead explants.
Experimental design and data analysis
The experiments were performed in three independent replications, with each replication containing 10 shoot tips. The experimental design was completely randomized. The results were expressed as a percentage of survived shoot tips to total shoot tips. The data were analyzed using Microsoft Excel 2013 and SAS v 9.2. After the normality transformation, the results were statistically analyzed by the analysis of variance (ANOVA), followed by the least significant difference (LSD) test for comparisons of different means of different treatments with the probability value set at p < 0.05. Statistics were presented as means with Standard Error of the proportion, and significant differences were denoted by different lowercase letters.
. Results
Cold storage
Before cold storage and cryopreservation, we tried to assess the type and optimal concentrations of plant growth regulators for the recovery medium. We found that the highest shoot multiplication was obtained in medium enriched with 4 mg l−1 BA. On the other hand, the largest number of root was produced on medium augmented with 0.3 mg l−1 NAA (data not shown). For slow-growth storage of C. rubra (L.) Rich., the encapsulated-dehydrated shoot tips (Figure 2) were stored at 4 °C for 6 months. Statistically significant differences were observed in the survival rate between the different pretreatments and the control (Table 2). All the pretreated shoot tips had better survival than the non-pretreated (control) explants after culture in the regeneration medium (Table 3). The encapsulation pretreatment increased the survival rates of shoot tips after storage at low temperature (4 °C). The highest survival rate (82.33%) was obtained in the shoot tips pretreated with encapsulation and air-desiccation for 2 h; however, no statistically significant difference in survival rates resulting from the encapsulation was observed, as the survival rate of the shoot tips after cold preservation by encapsulation was 77.66% (Table 3). The shoot tips stored at low temperature showed a 37.66% survival rate without any pretreatments, but this rate increased in the shoot tips pretreated with all treatments. The germination or survival rates in all the treatments were significantly higher than those in the control (Table 3). The encapsulation of the shoot tips together with the pretreatments with both 0.75 M sucrose and 2 h air-desiccation did not increase the survival rate than encapsulation and encapsulation together with 2 h air-desiccation after cold storage.
Figure 2
Cold storage of shoot tips of Cephalanthera rubra (L.) Rich., pretreated with encapsulation-dehydration. (A) Encapsulated shoot tips under sterile air-flow of laminar hood cabinet for air-desiccation (scale bars = 20 mm); (B) in vitro culture of shoot tips after 6 months of conservation (scale bars = 4 mm); (C) shoot tips growing on MS medium enriched with 4.00 mg l−1 BA after 60 days (scale bars = 7 mm); (D) shoot tips growing on MS medium enriched with 4.00 mg l−1 BA after 75 days (scale bars = 5 mm).
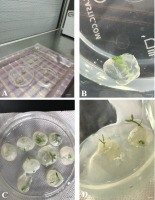
Table 2
Analysis of variance of the effect of different pretreatments on the survival percentage in Cephalanthera rubra (L.) Rich. after cold storage.
| Source of variance | df | Sum of squares | Mean square |
|---|---|---|---|
| Treatment | 7 | 4716.958333 | 673.851190** |
| Error | 16 | 132.666667 | 8.291667 |
| C.V. (%) | - | - | 4.93 |
Table 3
Mean comparison of the effect of different pretreatments on the survival percentage in Cephalanthera rubra (L.) Rich. after cold storage.
Cryopreservation
The ultra low temperature of LN is a drastic stress for biological materials. The current study revealed that all processes of the encapsulation-dehydration protocol, including encapsulation in the presence of 0.75 M sucrose and air-desiccation (Figure 3), were necessary to enhance the cryopreservation tolerance of C. rubra (L.) Rich. shoot tips. The explants encapsulated in alginate beads with or without sucrose showed higher survival after cryopreservation compared to the non-encapsulated shoot tips. There was a significant difference in the mean germination across the eight treatments when analyzed using an ANOVA test (Table 4). The survival percentage for the control and LN-recovered shoot tips ranged from 33.66 to 83.66% (Table 5). Minimum survival was observed in the control (non-pretreated) samples. The encapsulated shoot tips showed higher viability in the recovery medium compared to the non-encapsulated explants. For cryopreservation, the shoot tips stored in LN showed a 33.66% survival rate without any pretreatments, but this rate increased in the shoot tips pretreated with all the treatments (Table 5). The survival rates for all the treatments were significantly higher than those of the control. The highest germination percentage or survival rate (83.66%) after cryopreservation occurred when the samples were pretreated with encapsulation and air-desiccation (encapsulation-dehydration) for 2 h. There was no statistically significant difference in the survival rates between this treatment and the treatment with encapsulation solely, as the germination percentage of the shoot tips after cryopreservation by encapsulation was 68.66% (Table 5). The encapsulation of shoot tips together with the pretreatments with both 0.75 M sucrose and 2 h air-desiccation did not increase the survival percentage compared with encapsulation and encapsulation together with 2 h air-desiccation after cryopreservation.
Figure 3
Cryopreservation of shoot tips of Cephalanthera rubra (L.) Rich., pretreated with encapsulation-dehydration. (A) Encapsulated shoot tips under sterile air-flow of laminar hood cabinet for air-desiccation (scale bars = 10 mm); (B) in vitro culture of shoot tips immediately after cryopreservation and thawing (scale bars = 10 mm); (C) shoot tips growing on MS medium augmented with 4.00 mg l−1 BA after 60 days (scale bars = 7 mm); (D) shoot tips growing on MS medium augmented with 4.00 mg l−1 BA after 75 days (scale bars = 5 mm).
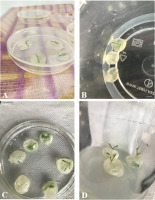
Table 4
Analysis of variance of the effect of different pretreatments on the survival percentage in Cephalanthera rubra (L.) Rich. after cryopreservation.
| Source of variance | df | Sum of squares | Mean square |
|---|---|---|---|
| Treatment | 7 | 4721.166667 | 674.452381** |
| Error | 16 | 188.666667 | 11.791667 |
| C.V. (%) | - | - | 6.06 |
Table 5
Mean comparison of the effect of different pretreatments on the survival percentage in Cephalanthera rubra (L.) Rich. after cryopreservation.
. Discussion
Some orchid species are facing extinction in the wild. Unfortunately, many tropical and subtropical species are in danger of extinction because of climate change as well as biotic and abiotic stresses. Germplasms of these plants should be preserved in every possible way for the presence and future. In the current study, shoot tips of C. rubra (L.) Rich. were encapsulated in sodium alginate beads, dehydrated in liquid medium enriched with a high concentration of sucrose, and desiccated under clean air-flow, then stored in cold conditions and plunged rapidly in LN. Optimum content of water in cells of plant materials is a critical stage in cryopreservation.
We tried to conserve the germplasm of C. rubra (L.) Rich., a threatened orchid species, by encapsulation-dehydration and showed its positive role. The present study demonstrated that the use of appropriate pretreatments can help the explants to withstand against low and ultra-low temperature for short- and long-term preservation, respectively. The non-pretreated shoot tips had lower survival after storage in the refrigerator and LN. Similar results have been reported on several ornamental species (Kaviani, 2011; Kaviani & Kulus, 2022; Kulus & Zalewska, 2014). Our results showed that the use of both chemical and physical dehydration in combination with each other reduced the regeneration rate of shoot tips after cold storage and cryopreservation. Contrary to our findings, a study on Buxus sempervirens showed the maximum survival (71.30 and 66.30%) after cold storage and cryopreservation of encapsulated explants dehydrated by 0.75 M sucrose and exposed to air-desiccation for 2 h (Negahdar et al., 2021). A combination of encapsulation, high sucrose concentrations, and air-desiccation as pretreatments before the exposure of germplasms to LN was effective in a range of endangered ornamental species (Kaviani, 2010, 2011; Kaviani & Kulus, 2022; Kulus & Zalewska, 2014; Teixeira da Silva et al., 2014). Differences in the content of intracellular water in various species and explants caused these different results. Thus, the concentration and incubation time of osmotic and physical dehydration may be manipulated. Preferably, 0.1–0.75 M sucrose can be used for a few hours for a few days. Also, the optimal physical dehydration (air-desiccation) duration is 4–6 h (Kaviani & Kulus, 2022; Kulus & Zalewska, 2014). The optimum pretreatment for encapsulated-dehydrated Paphiopedilum exul (Ridl.) Rolfe seeds was air-desiccation for 2 h under a laminar air-flow cabinet (Imsomboon & Thammasiri, 2020). Encapsulation is a proper technique as a pretreatment for cold- and cryo-storage of germplasms. Our work showed that the shoot tips encapsulated in alginate beads with or without sucrose had a higher survival rate after cold storage and cryopreservation compared to the non-encapsulated shoot tips. Similar findings were reported on B. sempervirens (Negahdar et al., 2021). Survival of encapsulated Cypripedium lentiginosum seeds stored at 5 °C decreased after 6, 12, and 24 months of storage, respectively (Jiang et al., 2017).
Some reports on ornamental plants, including endangered species, such as several cultivars of orchids, showed that non-encapsulated germplasm either did not survive or had very low survival after cryopreservation (Kaviani, 2011; Kaviani & Kulus, 2022; Kulus & Zalewska, 2014). It is increasingly apparent that cryopreservation, i.e. the storage of the germplasm at ultra-low temperatures (e.g., in liquid nitrogen), is required for the long-term and low-maintenance conservation of all types of orchid germplasms. Cryopreservation is the most efficient approach for the safe long-term conservation of rare and endangered ornamental plants like orchids (Kulus & Zalewska, 2014). Although almost all cryopreservation methods have been developed for several genera of the Orchidaceae family (Burkhan et al., 2022; Bustam et al., 2016; Kulus & Zalewska, 2014; Popova et al., 2016; Popova & Kim, 2019), this method for C. rubra species has not been documented before. Therefore, this study reported the first attempt of long-term storage of C. rubra using the encapsulation-dehydration method. Comparing the two methods tested for cryopreservation of the threatened orchid Cattleya labiata Lindley, it was observed that the regeneration of cryopreserved protocorms using encapsulation-dehydration was higher (42%) with 4 h dehydration in a laminar air-flow cabinet, whereas the use of the dehydration-vitrification procedure yielded 27% regeneration after dehydration for 10 min in a PVS2 solution (Galdiano & Lemos, 2018). The survival frequency of LN-stored PLBs of Vanda coerulea Griff. ex Lindl. without any pretreatments was 5% (Jitsupakul et al., 2011).
Production of beads around explants (encapsulation) reduces the pace of the dehydration process and allows the use of subsequent dehydration processes before cold storage and cryopreservation, which would be damaging or even lethal for non-encapsulated explants (Cruz-Cruz et al., 2013). Moreover, the alginate matrix provides enhanced physical protection of the explants from physical and oxidative stress during preservation (Teixeira da Silva et al., 2014). The presence of sucrose in the capsule, besides dehydration, may stimulate faster recovery of the explants after rewarming. Some reports revealed that the encapsulation-dehydration technique may provide better protection than other pretreatments (Kulus & Zalewska, 2014; Negahdar et al., 2021). This technique is most frequently used with ornamental plants, particularly those in danger of extinction (Kaviani & Kulus, 2022). Similarly, encapsulation of Begonia microshoots improved the survival rate after cold storage compared with non-encapsulated explants (Sakhanokho et al., 2013). Encapsulated somatic embryos of the orchid Vanda tessellate were successfully conserved at −4 °C for 12 months (Manokari et al., 2021). The beneficial encapsulation-dehydration technique was shown by some researchers in several endangered ornamental plants, such as the members of the Orchidacae family (Khoddamzadeh et al., 2011; Teixeira da Silva et al., 2014). Teixeira da Silva et al. (2014) showed that encapsulation-dehydration is a technique that provides a high survival frequency after cryogenic storage of Dendrobium germplasms.
The application of more advanced cryopreservation techniques, including encapsulation-dehydration, is becoming increasingly common for ornamental species (Merritt et al., 2014). Encapsulation of explants has some benefits, such as the ability to withstand against very harsh treatments, including dehydration, reducing the speed of the dehydration step and allowing the use of subsequent dehydration steps, reducing the moisture content of cells before immersion in LN, increasing the physical support of explants against mechanical and oxidative stress during storage, and accelerating pre- and post-storage steps in LN (Kaviani & Kulus, 2022; Ozden-Tokatli et al. 2008). Cryopreservation of some explants without encapsulation can be harmful and even fatal for them. In the present study, the positive role of encapsulation-dehydration as a protective pretreatment against LN stress was shown. The presence of sucrose in the beads, in addition to dehydrating, can stimulate faster regeneration of explants after thawing (Kaviani & Kulus, 2022). Our findings do not agree with these reports, because the regeneration of the cryopreserved shoot tips pretreated with encapsulation together with dehydration by both osmotic and air-desiccation was lower than that of encapsulated and pretreated with air-desiccation. Some reports showed that encapsulation-dehydration provided better protection of explants against cold and freezing stress than other techniques (Kaviani, 2011; Kaviani & Kulus, 2022). The encapsulation-dehydration technique has been widely used for ornamental plants (Kaviani & Kulus, 2022). The current study suggests the use of encapsulation-dehydration treatment for cryopreservation of C. rubra (L.) Rich. At present, one of the most successful shoot tip cryopreservation procedures is the encapsulation-dehydration method (Kaviani & Kulus, 2022; Zhang et al., 2023).
Orchid seeds and pollen are the most suitable propagules for cryopreservation of these plants due to their minimum size and less space requirements (Das et al., 2021; Kaur, 2019). For orchid seeds, the desiccation tolerance is common, but the longevity during storage is poor. The cryopreservation of orchid seeds shows promise, but some complexities in low-temperature storage behavior still require explanation and resolution. Protocorms and PLBs are probably most frequent explants used for cryopreservation of orchids. Studies on protocorms and PLBs mostly employ desiccation, vitrification, or encapsulation-dehydration (Das et al., 2021). Shoot tips and axillary buds were chosen as the plant materials due to the availability of the materials throughout the year and typically, plantlets regenerated via axillary buds or direct somatic embryogenesis are considered to be the most genetically uniform. The use of these explants has resulted in rapid multiplication of many orchids, especially those where there is failure of propagation through seeds. It also aids in successful establishment of clonal plants in forest habitats. Shoot tip culture is not considered economical in monopodial orchids since it leads to the arrest of growth and development of the mother plant; however, it can be used as a more reliable technique for tissue culture of sympodial orchids like Dendrobium (Das et al., 2021).
. Conclusion
In the present investigation, we tried to conserve an endangered orchid (Cephalanthera rubra (L.) Rich.) by in vitro techniques (cold storage and cryopreservation). More than one third of plant species are in danger of extinction. Conservation of plant biodiversity is necessary for plant breeding, genetic engineering programs, and sustainable development of valuable genotypes. The highest survival percentage after cold storage and cryopreservation was obtained with the encapsulation-air-desiccation technique. Future studies must be conducted toward finding of new approaches for safer plant germplasm preservation, particularly novel pretreatments.


