Introduction
Melatonin is a biogenic amine present in almost all life forms (Hardeland,2016; Tan et al.,2014). It was discovered in 1958 from the pineal gland of vertebrates (Lerner et al.,1958). In animals, it plays multifaceted regulatory roles in diverse processes, including circadian rhythm, homeostasis, seasonal photoperiodism, and immunological enhancement (Calvo et al.,2013; Galano et al.,2011; Maitra & Hasan,2016; Venegas et al.,2012). Although melatonin was first reported in plants about two decades ago (Dubbels et al.,1995; Hattori et al.,1995), it has received scientific attention only in recent years. Since its discovery in plants, melatonin has been established as a prominent signaling molecule and has inspired many researchers to examine its characteristics. Melatonin is a natural compound that not only enhances the growth and developmental processes, but also plays a prominent role in protecting plant life against various stressors (Arnao & Hernández-Ruiz,2015; Kołodziejczyk & Posmyk,2016; Nawaz et al.,2016; Posmyk & Janas,2009; Reiter et al.,2015; Wang et al.,2018; Zhang, Sun, et al.,2015). These properties characterize it as a biostimulator in plants (Arnao & Hernández-Ruiz,2014,2015; Janas & Posmyk,2013; Kołodziejczyk & Posmyk,2016). Moreover, it is worth mentioning that melatonin acts as a growth regulator and also as a powerful scavenger for different reactive oxygen species (ROS) and reactive nitrogen species. It plays a significant role in photosynthesis, photoprotection, senescence, apoptosis, and as an antioxidant. In addition, melatonin upregulates gene expression to deal with biotic and abiotic stresses. Thus, melatonin can be used as an effective growth regulator for sustainable crop production, without the risk of a negative impact on the external environment (Arnao & Hernández-Ruiz,2019; Beyer et al.,1998; Reiter et al.,2001,2002,2015; Rodriguez et al.,2004; Russel et al.,2002; Tan et al.,1993; Zhang et al.,2016).
This review aims to highlight the dynamic aspects of melatonin from the data available in the fields of biosynthesis, seed germination, plant growth and development, photosynthesis, and senescence. In addition, its role against various abiotic stresses, as an antioxidant, has been discussed. Furthermore, an effort has been made to shed light on its relationship with other phytohormones (viz., auxin, gibberellins, cytokinins, ethylene, abscisic acid, brassinosteroids, salicylic acid, and jasmonic acid) in the modification of plant growth and development.
Occurrence
Melatonin is a ubiquitous compound found in several species of plants (Table 1). In phototropic organisms, melatonin was first discovered in marine dinoflagellate Lingulodinium polyedrum (Balzer & Hardeland,1991; Pöggeler et al.,1989,1991), and later in macroalgae (Fuhrberg et al.,1996) and higher plants (Balzer & Hardeland,1996; Dubbels et al.,1995; Hattori et al.,1995). This indolamine is located mainly in roots, leaves, flowers, seeds, and bulbs of plant species belonging to families such as Poaceae, Rosaceae, Apiaceae, Vitaceae, and Brassicaceae. Melatonin concentration varies with the species and stage of plant development, and also with external environmental factors (Byeon & Back,2014; Feng et al.,2014; Hardeland,2016; van Tassel et al.,2001; Zhao et al.,2013). Under identical conditions, corn (Zea mays) and rice (Oryza sativa) seeds exhibited the highest values of melatonin, ranging from 11 to 2,034 ng g−1 dry weight (DW) and 11 to 264 ng g−1 DW, respectively (Wang et al.,2009). Chen et al. (2003) quantified the melatonin level in 64 medicinal herbs and reported concentrations ranging from 12 to 3,771 ng g−1 DW. Among plants, to date, the highest level (227–233 µg g−1 DW) of melatonin has been detected in different varieties of pistachio (Pistacia vera) kernels (Oladi et al.,2014). Although Arabidopsis thaliana is not of major agronomic significance, it offers significant advantages for basic research in genetics and molecular biology. Melatonin has been detected in A. thaliana leaves at a level of 80–120 ng g−1 DW; however, it did not show any significant variations during the day (Hernández et al.,2015). Tan et al. (2007) reported high levels of melatonin closer to sunset in water hyacinth (Eichhornia crassipes) leaves (34–72 ng g−1 fresh weight – FW; 2.3–3.6 ng g−1 FW at night] due to the promotion of melatonin synthesis by light. Similar results were also observed for morning glory (Pharbitis nil) and tomato (Solanum lycopersicum) (van Tassel et al.,2001).
Endogenous melatonin values increased in plants under unfavorable biotic and abiotic conditions. Rice seedlings exposed to darkness and high temperature exhibited 2.9–4.9 ng g−1 FW more melatonin, which was directly correlated with the activity of its biosynthetic enzymes (Byeon & Back,2014). A similar pattern of increase in melatonin level (more than 135%) was exhibited by tomato plants exposed to shade (Riga et al.,2014).
Table 1
Content of melatonin in particular plants.
Biosynthesis
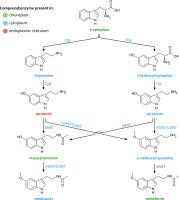
Melatonin is an indolic compound synthesized from serotonin in both plants and animals, with a minor difference in the two synthetic pathways. Melatonin is synthesized from tryptophan (an essential aromatic amino acid) through various four-step reactions catalyzed by six enzymes, i.e., tryptophan decarboxylase (TDC), tryptophan hydroxylase (TPH), tryptamine 5-hydroxylase (T5H), serotonin N-acetyltransferase (SNAT), N-acetylserotonin O-methyltransferase (ASMT), and caffeic acid O-methyltransferase (COMT) (Figure 1). Multiple synthetic pathways exist, and all of them involve serotonin as an intermediate. Under normal growth conditions or low cellular serotonin levels, the following pathways exist: (i) tryptophan / tryptamine / serotonin / N-acetylserotonin / melatonin; (ii) tryptophan / 5-hydroxytryptophan / serotonin / N-acetylserotonin / melatonin. On the other hand, the pathway: tryptophan / tryptamine / serotonin / 5-methoxytryptamine / melatonin occurs when plants produce large amounts of serotonin, for example, during senescence. Tryptophan is first converted into tryptamine catalyzed by the enzyme TDC, which is then hydroxylated by T5H into serotonin (5-hydroxytryptamine) (Back et al.,2016; Kang et al.,2008,2010,2011,2013; Park et al.,2012). Thereafter, serotonin is N-acetylated by SNAT, which is further methylated by ASMT (or together with COMT) to synthesize melatonin (Champney et al.,1984; Lei et al.,2013). TDC action is considered as the rate-limiting step in the pathway of melatonin biosynthesis (Byeon et al.,2014,2015; Lee et al.,2014), suggesting that two alternate routes may be involved in methylation. Moreover, SNAT has been reported to metabolize serotonin directly into N-acetylserotonin through alternative minor pathways. ASMT and COMT catalyze the conversion of serotonin into 5-methoxytryptamine, which is then converted into melatonin by SNAT. The melatonin biosynthetic capacity associated with the conversion of tryptophan to serotonin is considerably higher than that associated with the conversion of serotonin to melatonin, which yields a low level of melatonin synthesis in plants. Lee et al. (2018) also found that exogenous N-acetylserotonin can be converted to serotonin in rice seedlings by N-acetylserotonin deacetylase (ASDAC), which may result in a reduction in the content of melatonin.
Melatonin intermediates are produced in various subcellular locations, such as the cytoplasm, endoplasmic reticulum, and chloroplasts (Figure 1). Depending on the pathway, the final subcellular site of melatonin synthesis can be the cytoplasm or the chloroplasts, which may differentially affect the mode of action of melatonin in plants (Tan et al.,2016; van Tassel et al.,2001; Wei et al.,2016,2017). SNAT is found in both chloroplasts and mitochondria. TPH, ASMT/COMT, and TDC are distributed in the cytoplasm, while T5H is found only in the endoplasmic reticulum. These subcellular locations of melatonin synthesis enzymes suggest that during evolution, the sites of melatonin synthesis became more diverse and extended to the cytoplasm and endoplasmic reticulum (Back et al.,2016; van Tassel et al.,2001).
Physiological Role
Seed Germination
Successful germination and seedling establishment are the two major steps for establishing plant populations. Exogenous application of melatonin to seeds improved the germination rate as well as germination percentage in lentils (Lens culinaris) and kidney beans (Phaseolus vulgaris) (Aguilera et al.,2015), Glycine max (Wei et al.,2015), Pisum sativum (Szafrańska et al.,2016), and Cucumis sativus (Posmyk, Bałabusta, & Janas,2009; Posmyk, Bałabusta, et al.,2009; Zhang et al.,2013; Zhang, Zhang, et al.,2014; Zhang, Zhang, et al.,2017). In the natural course, during the initial stages of seed germination, melatonin concentration increases rapidly and attains a peak after 14 hours. However, its concentration decreases as germination proceeds and attains a steady state during the later stages of seedling establishment. Cucumber seeds primed with 1 µM melatonin have about 9 times more melatonin during the first day of germination, which then decreases considerably by alleviating the inhibitory effect of salinity. The initial increase of melatonin in cytoplasm might contribute to the mobility of nutrients to the metabolic site of germinating seeds (Aguilera et al.,2015). This increase may also be due to a reduction in the oxidative burst due to the enhancement of antioxidant activity, including activities of enzymes like catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD), with a consequent 1.4–2-fold increase in CsCAT, CsCu-ZnSOD, CsFe-ZnSOD, and CsPOD. Melatonin alters seed germination by decreasing the ABA content, as it downregulates the rate-limiting genes related to ABA biosynthesis (e.g., 9-cis-epoxycarotenoid dioxygenase, CsNECD2, 0.29-fold). At the same time, gibberellin (GA) biosynthesis associated genes (e.g., gibberellin 3β-dioxygenase – GA3ox; gibberellin 20-oxidase – GA20ox) are upregulated by melatonin, causing a sudden accumulation of GA. Hence, it is assumed that melatonin influences signal transduction and plays a role in inducing seed germination processes. The positive effects of presowing melatonin treatment are related not only to seed quality, but also to seedling development, growth, and plant yield (Huang et al.,2018; Janas et al.,2009; Posmyk, Bałabusta, & Janas,2009; Szafrańska et al.,2013,2014; Zhang, Zhang, et al.,2014).
Melatonin has been established as a promising agent for enhancing seed germination. Various reports demonstrate its positive effect in a dose-dependent manner; however, the effect differs from species to species. Lower concentrations (5 and 20 µM) of melatonin caused a significant increase in the germination of Stevia rebaudiana seeds as compared to higher concentrations (100 and 500 µM) (Simlat et al.,2018). Posmyk et al. (2008) also reported that lower concentrations (1 and 10 µM) significantly enhanced the germination rate in red cabbage. Hence, at lower concentrations, melatonin increases the germination rate, germination index, germination potential, and germination survival rate, whereas these are inhibited by higher doses (Gao et al.,2018). Similarly, Korkmaz et al. (2017) revealed that melatonin (1 or 5 µM) application in pepper seeds enhanced their germination and seedling emergence performance, in chilling stress conditions. This may be due to the induced synthesis of various antioxidants that protect the stressed plants from the oxidants and reduce the germination emergence period of the seedlings. Conversely, higher concentrations have been shown to exert inhibitory impacts (Chen et al.,2009; Hernández-Ruiz et al.,2004; Wei et al.,2015).
The Possible Pathway of the Effect of Melatonin on Seed Germination
Successful seed germination is crucial for plant growth and development. Xiao et al. (2019) reported that a low concentration of melatonin promoted seed germination in cotton (Gossypium hirsutum) by enhancing the germination potential, germination rate, final fresh weight, vigor index, germination index, and mean germination time. Moreover, melatonin regulates GA3 and ABA contents in germinating seeds by decreasing the ABA (68%) content and increasing the GA3 content by about 1.7–2.5 times. In another study, Limonium bicolor plants treated with melatonin also showed high levels of melatonin and GA3, low levels of ABA, and a high level of amylase and α-amylase activity. Melatonin upregulated the expression of GA biosynthesis (GA20ox and GA3ox) genes, downregulated key genes involved in ABA biosynthesis (LbNCED1 and LbNCED3), and upregulated ABA 8′-hydroxylase genes (LbCYP707A1 and LbCYP707A2) (Li et al.,2019). In addition, it upregulated the enzymes involved in glycolysis, citric acid cycle, and the glyoxylate cycle (isocitrate lyase and malate synthetase). It also upregulated the activities of α- and β-amylases and promoted starch catabolism for ATP production in germinating seeds. These results demonstrate that melatonin acts as a positive regulatory factor in energy metabolism and seed germination (Zhang, Zhang, et al.,2017).
Growth and Development
Aguilera et al. (2015) reported that melatonin considerably enhanced root growth in kidney bean and lentil sprouts. Their results show that the application of 20 µM melatonin increases radicle growth in lentils by about 1.4 times when compared to test seedlings treated with water (control). However, seedlings of kidney beans treated with melatonin increased by about 1.6 times compared to the control seedlings. This impact of melatonin depends not only on its concentration but also on the species and the stage of growth and development (Chen et al.,2009). At lower concentrations, melatonin treatment enhanced the number of roots, and altered the rooting percentage and root length in in vitro cultures of sweet cherry. However, higher concentrations had a negative effect, resulting in restrained growth and development of cherry roots (Sarropoulou, Dimassi-Theriou, et al.,2012; Sarropoulou, Therios, et al.,2012). Similarly, Stevia seedlings treated with lower concentrations of melatonin showed significant improvement in growth compared to those treated with higher concentrations (Simlat et al.,2018). Kang et al. (2010) also confirmed the positive role of melatonin while working on transgenic rice plants and in vitro cherry tomato cultures.
Melatonin is known to alter plant growth and development in a dose-dependent manner. In Stevia plantlets, melatonin enhanced growth and development of aboveground parts, viz., an increase in fresh weight, stem length, and number of leaves was observed (Simlat et al.,2018). In A. thaliana, melatonin at an optimal concentration (10–30 μM) increased fresh weight than at higher concentrations (200–400 μM) (Bajwa et al.,2014). Moreover, melatonin pretreatment mitigated the drought-stressed inhibition of naked oat seedlings by promoting seedling growth and plant height (2.3%), and increasing stem thickness (14.5%), plant fresh weight (10.7%), and dry weight (7.6%) when compared to untreated seedlings (Gao et al.,2018). However, at a higher dose of 500 µM, it led to toxicity by inhibiting all growth parameters. Inhibition of growth and development was also observed in red cabbage under a higher concentration (100 µM) of melatonin (Posmyk et al.,2008), which might be due to oxidative protein degradation and other issues related to protein biosynthesis and antioxidant activity (Kładna et al.,2003). To summarize, melatonin is now known to improve various plant characteristics, including germination, growth (Kładna et al.,2003), and grain yield (Byeon & Back,2014).
Effect on Photosynthesis
Photosynthesis is sensitive to various stresses (biotic and abiotic), which exert a negative effect by decreasing the chlorophyll content, destroying excitons in chloroplasts, and stomatal closure. However, the application of melatonin is beneficial in alleviating these adverse effects of stress. Melatonin improves the efficiency of photosystems at a cellular level by lowering the rate of chlorophyll degradation, and increasing stomatal conductance, aiding in both opening and closing of stomata; therefore, plants exhibit a higher rate of photosynthesis and CO2 assimilation as well as an increase in antioxidant levels (Arnao & Hernández-Ruiz,2009,2014; Li, Zeng, et al.,2018). Melatonin protects against chlorophyll degradation, and hence, improves photosynthetic efficiency. It also reduces the negative impacts of abiotic stresses, guards/protects the chloroplast, promotes sugar assimilation, and increases proline accumulation to maintain normal cellular functions under stress. This suggests that melatonin may have a role as a marker of stress tolerance through an osmoregulatory response (Arnao & Hernández-Ruiz,2014,2015). Moreover, melatonin in tomato (Lycopersicon esculentum) enhances quantum yield by mitigating photo-oxidative damage, and by facilitating the repair of photo-oxidatively damaged D1 protein (Zhou et al.,2016). Melatonin also stimulates the genes related to photosynthesis, carbon assimilation, and ascorbate biosynthesis (Arnao & Hernández-Ruiz,2015). Sarropoulou, Dimassi-Theriou, et al. (2012) also reported that melatonin treatment of stressed cherry rootstock resulted in an increase in photosynthetic pigments, ultimately leading to higher carbohydrate accumulation and an increase in biomass production and proline content. Similarly, salt-stressed soybean plants responded to melatonin with an improvement in growth, yield, and tolerance against stress. This enhancing effect of melatonin is attributed to the induction of expression of genes associated with photosynthesis, carbohydrate/fatty acid metabolism, and ascorbate biosynthesis. In soybean, melatonin significantly upregulates PsaK and PsaG of photosystem I, oxygen-evolving enhancer proteins PsbO and PsbP, the ferredoxin gene PetF, and the VTC4 gene involved in ascorbate biosynthesis (Wei et al.,2015). Moreover, Han et al. (2017) reported that melatonin-pretreated seedlings of rice (O. sativa) exhibited a significant increase in the photosynthetic pigment (chlorophyll and carotenoid) content under stress conditions. In addition, optimal stomatal conductance and increased intercellular CO2 accumulation led to an increase in net photosynthetic rate, improved water use efficiency, and enhanced efficiency of PSII, by increasing PSII proteins (D1, CP43, Lhcb1, and Lhcb2), under stress conditions. Comparable observations of photosynthetic improvement were recorded in bermudagrass (Cynodon dactylon) (Hu et al.,2016), A. thaliana (Bajwa et al.,2014), and wheat (Triticum aestivum) (Turk et al.,2014). It may, therefore, be suggested that melatonin promotes growth by enhancing photosynthesis via the upregulation of expression of photosynthesis-associated genes (Arnao & Hernández-Ruiz,2014; Hardeland,2015).
Role of Melatonin in Reproductive Development and Circadian Rhythms
Melatonin is a promising candidate for mediating the circadian process by regulating the circadian clock and rhythmic changes in animals (Cassone,1998). After its discovery in plants, it was believed to have a comparable function as recognized in mammals. Thus, initial research focused on examining its potential role in circadian rhythms and the associated aspects (flowering, photoreceptors, vernalization, hormones, and circadian rhythms) (Arnao & Hernández-Ruiz,2006; Rodriguez et al.,2004; Zhang, Sun, et al.,2015; Zhao et al.,2019).
Kolář et al. (1997) noticed fluctuations in endogenous melatonin concentrations in Chenopodium rubrum during 12-hr light/dark cycles. The variations were insignificant during the day, whereas a significant rise in melatonin levels was observed during the dark period. Such an increase during the dark period was similar to that reported in mammals. Furthermore, the macroalga Ulva sp. exhibits a melatonin rhythm with a peak at night in a long-photoperiod day (16 hr) (Tal et al.,2011). Further, under natural conditions, water hyacinth (E. crassipes), exhibited the highest concentration of melatonin with a peak occurring late in the light phase (Tan et al.,2007). In another study, P. nil grown under light/dark photoperiod showed no significant change in endogenous melatonin concentration. Moreover, Lycopersicum esculentum investigated at different ripening stages did not show any significant differences in melatonin content (van Tassel et al.,2001).
Antioxidative Defense System
The key function of melatonin in plants is to act as an antioxidant and protect against various environmental pollutants (Arnao & Hernández-Ruiz,2019; Manchester et al.,2000; Nawaz et al.,2016; Tan et al.,1993). Arnao and Hernández-Ruiz (2009) exposed barley (Hordeum vulgare) plants to multiple stresses (zinc, hydrogen peroxide, or NaCl) and reported an upsurge in endogenous melatonin level. This increase in the melatonin level was dependent on the age of the plant and stress level. The same results have been noted in lupin (Lupinus albus) seedlings. Moreover, melatonin application to plants (barley and lupin) improved plant growth and development as well as plant survival under multiple stresses. In addition, pea and red cabbage seedlings exposed to melatonin under copper stress exhibited similar responses (Posmyk et al.,2008). In their study on the mitigative effect of melatonin in apple seedlings (45 days old) subjected to salinity stress, Li et al. (2012) reported a decline in the inhibitory effect on shoot height, leaf number, chlorophyll content, and electrolyte leakage, compared to water treated seedlings. Furthermore, hydrogen peroxide levels were drastically reduced, ROS metabolizing enzymes (ascorbate peroxidase, APX, CAT, and peroxidase activity) were activated, and both Na+ and K+ transporters (NHX1 and AKT1) were upregulated, which reduced salt-induced stress in melatonin-rich transgenic rice (Park et al.,2013).
Moreover, melatonin application enhanced antioxidant enzyme activity in drought-stressed naked oat (Avena nuda) seedlings. Following 100 μM exogenous melatonin treatment of drought-stressed oat seedlings, the activity of SOD, POD, CAT, and APX was improved by 110.4%, 34.3%, 26.1%, and 10.4%, respectively (Gao et al.,2018). Martinez et al. (2018) used a combination of salinity and heat to evaluate the total antioxidant capacity of leaf extracts of tomato seedlings in the presence and absence of melatonin. Their results showed an 85% loss in protective antioxidant levels in stressed seedlings deprived of melatonin, while melatonin-treated seedlings exhibited only a 30% loss, compared to control plants. In addition, stressed seedlings treated with melatonin also showed lesser accumulation of H2O2. At lower concentrations, melatonin may act as either a hydrophilic or hydrophobic antioxidant. This property allows it to move rapidly and freely between cell compartments to shield them against reactive oxygen species (Venegas et al.,2012). Melatonin reduces the oxidative burst of various biomolecules, such as nucleic acids, proteins, and lipids. In summary, the protective properties of melatonin are: (i) it acts as a free radicle scavenger, (ii) enhances activity/accumulation of antioxidant enzymes, (iii) changes the redox status of the cell via regulation of glutathione synthesis and oxidation, (iv) ensures protection of antioxidant enzymes from oxidative destruction, (v) reduces free radicle formation and electron leakage by enhancing the efficiency of mitochondrial electron transport chain. These characteristics make melatonin a potent and effective regulator preventing or nullifying oxidative stress (Beyer et al.,1998; Li, Brestic, et al.,2018; Reiter et al.,2002; Rodriguez et al.,2004; Wang et al.,2012).
Senescence
Since the discovery of melatonin from the bovine pineal gland, this indolamine has been known to neutralize age-related impairments, and under certain experiments, to prolong the life-span of some animals to a certain extent (Tan et al.,2018). As aging is not directly associated with plants, its role in senescence has been studied, and it has been confirmed as an antisenescence compound. Liang et al. (2018) reported that exogenous application of melatonin (200 µm) in kiwifruit delayed senescence. Their results further showed that melatonin efficiently promoted the transcription of CAB genes, declined chlorophyll degradation, favored the accumulation of soluble sugars and proteins, and maintained normal cell metabolism. Moreover, an increase in the content of flavonoids and antioxidant substances (ascorbic acid – AsA, glutathione – GSH), and a decrease in the malondialdehyde (MDA) content had a positive effect on maintaining the cell membrane, validating its role in delaying leaf senescence.
Weeda et al. (2014) showed that melatonin treatment upregulated genes involved in ABA, salicylic acid (SA), ethylene, and jasmonic acid (JA) biosynthetic pathways, and downregulated genes associated with both cell wall synthesis and modifications and auxin signaling in A. thaliana. Most of the SA, JA, ABA, and ethylene-responsive genes induced by melatonin are also induced in response to stresses. Byeon and Back (2014) reported that melatonin downregulates aging-associated genes, genes encoding JA-induced protein, senescence-associated protein 29 (SAG29), and polygalacturonase in rice seedlings. Similar results, pertaining to the protective role of melatonin against chlorophyll degradation, have also been reported in leaf sections of barley (Arnao & Hernández-Ruiz,2009). Furthermore, various studies have shown a downregulation of the senescence-associated gene 12 (SAG12), and few variations in the influence on redox parameters, like lowering of H2O2 levels, reduction in GSH content, the elevation of ascorbate content, and elevated activity of redox-related enzymes, such as APX, glutathione reductase, CAT, and POD (Byeon & Back,2014; Wang, Sun, Chang, et al.,2013). Moreover, melatonin inhibited the expression of the sugar-sensing and senescence-associated hexokinase-1 gene (HXK1), and upregulated various autophagy-related genes (ATGs namely ATG3, ATG7a, ATG7b, ATG8g, ATG8h, ATG9, ATG10, and ATG18a) during the terminal stage of senescence (Wang, Sun, Chang, et al.,2013). Melatonin was shown to regulate the expression of senescence-associated genes, such as PAO, HXK1, SAG12, and ATGs. These properties highlight the senescence-delaying effects of melatonin in plants (Hardeland,2015). Therefore, melatonin prevents senescence, either by upregulating the expression of senescence-preventing genes or by downregulating the expression of senescence-promoting genes.
Role of Melatonin Under Abiotic Stress
Various studies have reported melatonin concentrations under natural and modified conditions, and have also explored its enormous potential and diverse roles in plants. Its vital role as an antistress agent against abiotic stresses, such as drought conditions, salinity, low, and high ambient temperatures, UV radiation, and toxic chemicals, as well as biotic stresses, has been revealed. Melatonin-treatment had a positive effect on its accumulation in plants, and upregulated the antistress genes under abiotic stresses (viz., cold, drought, osmotic stress), indicating its involvement in abiotic stress signaling (Wang et al.,2018; Zhang, Sun, et al.,2015).
Drought/Water Tolerance
Wang, Sun, Li, et al. (2013) reported that melatonin treatment reduces drought-induced stress in apple plants, by improving the efficiency of photosystem II, via mitigation of the stress-induced inhibition of photosynthesis, and maintaining a higher level of CO2 assimilation and stomatal conductance. In another experiment with cucumber seedlings treated with melatonin, a decline in chlorophyll degradation along with an increase in the photosynthetic rate and antioxidant enzymes was observed, which reduced the inhibitory effects of water stress (Zhang et al.,2013). Exogenous application of melatonin relieved rapeseed seedlings from the negative impact of drought stress on growth, and substantially increased the leaf area, and root and shoot biomass. The plants accumulated more soluble sugars and proteins and significantly less H2O2. Moreover, melatonin significantly increased the activity of APX, CAT, and POD under drought stress (Li, Zeng, et al.,2018). Similarly, melatonin treatment neutralized the impact of drought stress on seed germination and root viability. It also increased net photosynthesis and chlorophyll content (Zhang et al.,2013). In another study on wheat seedlings, melatonin treatment remarkably reduced drought stress, promoting the accumulation of antioxidant enzymes and decreasing ROS formation. It also reduced membrane damage, maintained the grana lamella, and protected chloroplast and leaf structure. Melatonin enhanced the photosynthetic rate and efficiency of photosystem II, and the activity of enzymes and the accumulation of transcripts related to GSH and AsA function (Cui et al.,2017). Moreover, melatonin-mediated lateral root formation in Malus species improved the water absorption capacity (Li et al.,2015).
Salinity Tolerance in Plants
Salt stress is one of the most common challenges that hinder plant growth and development. Salt stress leads to water scarcity and damages various physiological functions in plants (Parida & Das,2005). Various studies have reported the role of melatonin in reducing the adverse effects of salt stress in plants by its effect on the salt-tolerant genes. In rice seedlings experiencing salt stress, melatonin treatment induced salt tolerance by delaying cellular senescence and apoptosis, reducing chlorophyll degradation, downregulating senescence-related genes, enhancing antioxidant enzyme activity, and decreasing H2O2 accumulation. Thus, overall plant health was significantly improved (Liang et al.,2015). Similarly, melatonin treatment mitigated salt-induced growth inhibition in wheat seedlings, as evident from increased biomass of shoots, higher chlorophyll level, enhanced photosynthetic rate, and photosystem II efficiency. The plants also exhibited a reduction in the accumulation of hydrogen peroxide (H2O2), which suggests a definitive role of melatonin in overcoming salt stress in plants. It is worth emphasizing that melatonin can be regulated via the positive-feedback loop and an increase in the concentration of endogenous melatonin levels by the activation of the TaSNAT transcript, encoding a key enzyme in melatonin biosynthesis (Ke et al.,2018). In addition, exogenous melatonin increased salt tolerance in watermelon, by neutralizing its adverse effects on photosynthetic rate and reducing the accumulation of ROS (Li et al.,2017). Seed germination in cucumber was also promoted by melatonin through the promotion of energy production (Zhang, Zhang, et al.,2017).
Heavy Metal Tolerance
Heavy metal contamination is a severe environmental problem for all organisms, and particularly in plants. However, melatonin application appears to have a beneficial effect on plant tolerance to different metal stresses. Cucumber seedlings pretreated with exogenous melatonin not only exhibited an improved endogenous melatonin concentration, but also showed a positive response in alleviating nitrate-induced growth retardation (Zhang, Sun, et al.,2017). Melatonin application was found to upregulate the transporters, viz., ABC transporter, PDR8 (PLEIOTROPIC DRUG RESISTANCE 4), and HMA4 (HEAVY METAL ATPASE 4), leading to a decline in the accumulation of cadmium and an improvement of the operating redox imbalance in plants (Gu et al.,2017). Similarly, in watermelon, melatonin eased vanadium stress and increased chlorophyll content and sugar accumulation, and positively affected both growth and development. An increase in chlorophyll content, shoot biomass, and antioxidant enzyme activity, as well as a reduction in cadmium accumulation, was observed in Malachium aquaticum and hyper-accumulator Galinsoga parviflora by melatonin application (Tang et al.,2018). In addition, melatonin increased the photosynthetic efficiency, chlorophyll content, and activity of enzymes related to carbon assimilation in wheat seedlings exposed to nano-ZnO (Zuo et al.,2017). The positive role of melatonin in alleviating metal toxicity has been reported in other studies, e.g., mitigation of aluminum toxicity in soybean seedlings (Zhang, Zeng, et al.,2017), protecting red cabbage seedlings against toxic effects of copper ions (Posmyk et al.,2008), and improvement of cadmium tolerance in tomato plants (Li, Hasan, et al.,2016). Moreover, Hasan et al. (2015) reported that melatonin treatment eases the toxicity caused by cadmium stress in tomato plants by enhancing antioxidant enzyme activity, directly relieving plants from the oxidative burst, augmenting their growth and development, and increasing the photosynthetic rate and accumulation of photo-assimilates. Melatonin also facilitated the repair of damage caused by photooxidation, by enhancing the turnover/synthesis of D1 proteins (Zhou et al.,2016). Recent studies have revealed that melatonin stimulates the expression of genes involved in photosynthesis, carbon assimilation, fatty acid metabolism, and ascorbate biosynthesis (Arnao & Hernández-Ruiz,2015).
Melatonin is known to alleviate environmental stress by its well documented free radical scavenging activity, also during heat stress. Exogenous melatonin improved thermal tolerance in kiwifruits by alleviating the H2O2 content, which might be due to its electron neutralizing property and upregulation of genes involved in the synthesis of antioxidant enzymes like SOD, CAT, and POD, which ultimately decrease the content of reactive oxygen species and H2O2 (Arnao & Hernández-Ruiz,2019; Galano,2011; Galano et al.,2011; Reiter et al.,2002). Similar results were also observed in C. sativus (Zhao et al.,2016), C. dactylon (Shi et al.,2015), and Malus sp. (Wang, Sun, Chang, et al.,2013). Ahammed et al. (2019) described the role of endogenous melatonin in tomato under high heat conditions by silencing the CAFFEIC ACID O-METHYLTRANSFERASE 1 (COMT1) gene involved in melatonin formation and lignin synthesis. This silencing of the melatonin biosynthetic gene resulted in the induction of oxidative stress and an increase in the electrolyte leakage percentage and malondialdehyde concentration, followed by a decrease in the activity of key antioxidant enzymes, viz., APX and CAT, the main regulators of thermotolerance in plants. Moreover, the exogenous application of melatonin improved the level of endogenous melatonin, effectively mitigating the oxidative stress-induced under adverse effects of heat. Under global warming predictions, melatonin may be of potential use in neutralizing the impact of higher temperatures for better crop productivity.
Relation with Other Phytohormones
The growth-promoting activity of melatonin shows a similarity with that of the known phytohormone auxin. In various experimental studies, melatonin has been shown to enhance the growth of both aerial as well as underground parts, in Prunus, Triticum, Brassica, Hordeum, Avena, Lupinus, Arabidopsis, Oryza, Helianthus, Punica, Cucumis, Solanum, Glycine, and Zea plants (Arnao & Hernández-Ruiz,2017). Its growth-enhancing capacity is evident even under unfavorable conditions, e.g., as shown in Helianthus annuus, Z. mays, A. thaliana, C. dactylon, and Malus sp. (Kim et al.,2016; Li, Liang, et al.,2016; Mukherjee et al.,2014; Shi et al.,2015). Melatonin improves stem growth and root regeneration by up to 3 or 4 times in plants like Lupinus, Phalaris, Triticum, Hordeum, Arabidopsis, and Cucumis, in comparison to control seedlings (Arnao & Hernández-Ruiz,2017). Growth-promoting activity of melatonin has also been demonstrated in wheat (Turk et al.,2014), perennial ryegrass (Zhang, Shi, et al.,2017), cucumber (Zhang, Zhang, et al.,2017), pepper (Korkmaz et al.,2017), lentil, and bean (Aguilera et al.,2015). Melatonin treatment has been shown to increase auxin concentration by 1.4–2.0 times in Brassica juncea (Chen et al.,2009) and tomato plants (Wen et al.,2016). Conversely, in transgenic plants, its application decreased the endogenous auxin concentration by about 7 times in tomato and by about 1.4 times in A. thaliana. Exogenous melatonin in transgenic lupin plants developed auxin-like responses, such as root growth and rhizogenesis, increase in adventitious roots, apical dominance (Wen et al.,2016), and more lateral adventitious roots, by modifying the order of root arrangement/distribution, time interval, number, and length (Arnao & Hernández-Ruiz,2007). Transgenic A. thaliana contains 2–4 times more melatonin than the wild type. This increment in melatonin level is caused by the transfer of Malus zumi N‐acetylserotonin‐O‐methyltransferase (MzASMT1) gene from drought-stressed apple plants (Arnao & Hernández-Ruiz,2007; Koyama et al.,2013). Similar results were also observed in tomato plants (Wen et al.,2016), cucumber (Zhang, Sun, et al.,2017), pomegranate (Sarrou et al.,2014), and sweet cherries (Kang et al.,2010; Murch et al.,1997). Furthermore, Chen et al. (2009) reported that application of exogenous melatonin increased the auxin concentration in Brassica juncea. This suggests that melatonin has similar effects to indole-3-acetic acid (IAA), in both adventitious and lateral root induction (Arnao & Hernández-Ruiz,2014).
It must be noted that though the auxin-like the role of melatonin is well documented, evidence suggests that this is a result of the depletion of the substrate pool – as tryptophan serves as the substrate for both IAA and melatonin biosynthesis pathways (Arnao & Hernández-Ruiz,2015; Perez-Llorca et al.,2019). Wang et al. (2014) reported that the upregulation of an ovine AANAT transgene in ‘Micro-Tom’ tomato plants triggered an increase in the melatonin level. However, a decline in the auxin level was noted, which was ultimately correlated with the loss of apical dominance.
Regarding its comparability with GA, the application of exogenous melatonin alters GA level by upregulating genes associated with GA biosynthesis, such as GA20ox and GA3ox, leading to greater accumulation of GA4 (Zhang, Zhang, et al.,2014). Another important feature of melatonin is its involvement in gravitropism of underground tissues. Arnao and Hernández-Ruiz (2017) demonstrated that the disruption of natural auxin level by melatonin-enriched agar blocks gave rise to lateral roots with a loss of verticality. They concluded that melatonin induces negative gravitropism. Application of melatonin downregulates the darkness-induced senescence process by reducing chlorophyll loss in barley leaves, in a dose-dependent manner, similarly to cytokinins, but at a relatively slower pace (Arnao & Hernández-Ruiz,2009). Similarly, the impact of melatonin on the process of senescence has been reported in various other species, including ryegrass (Gu et al.,2017; Zhang, Shi, et al.,2017), cassava tuber (Hu et al.,2018; Ma et al.,2016), sunflower (Mukherjee et al.,2014), rice (Liang et al.,2015), Malus domestica (Wang et al.,2012), and C. sativus (Zhang et al.,2013). Application of melatonin in A. thaliana (Weeda et al.,2014) and Malus hupehensis (Wang et al.,2012) has been shown to downregulate chlorophyll-degrading enzymes.
Zhang et al. (2016) observed that melatonin downregulated senescence-associated genes (LpSAG12 and Lph36) in Lolium perenne, which delayed senescence and favored growth, and improved tiller number, cell membrane stability, chlorophyll retention, and photosynthesis, in addition to increasing the level of cytokinin precursors, such as isopentenyladenine and trans-zeatin riboside, under heat stress. Cytokinin biosynthetic pathway genes (LpIPT2 and LpOG1) were also activated under unfavorable stress conditions. In the case of ABA, melatonin treatment decreases its content by downregulating the key enzyme (9-cis-epoxycarotenoid dioxygenase), involved in ABA biosynthesis. Furthermore, melatonin can trigger the overexpression of genes associated with ABA catabolism (Huang et al.,2018). Pretreatment of drought-stressed apple seedlings with melatonin decreased the ABA concentration by half, which was attributed to the alteration of expression of genes involved in ABA biosynthesis or catabolism (Li et al.,2015).
Melatonin treatment upregulates 1-aminocyclopropane-1-carboxylic acid (ACC) synthase expression, leading to an increase in ethylene generation. In addition, melatonin regulates NR (never-ripped) and ETR4 (ethylene receptor 4), ethylene receptor genes, as well as LeEIL1, LeEIL3, and LeERF2 (encoding ethylene-transducing factors) (Sun et al.,2015). Upregulation of genes related to ACC synthase was also observed in melatonin-treated A. thaliana (Weeda et al.,2014). In tomato plants treated with melatonin, the expression of cell wall modifying proteins, including polygalacturonase, pectin esterase 1, β-galactosidase, and expansin 1, was upregulated. Melatonin also increased ethylene production, which was correlated with altered ACC synthase expression. This suggests that melatonin can influence ethylene to regulate textural changes in tomato fruit (Sun et al.,2015).
Melatonin Signaling Under Stress Conditions in Plants
Previous studies have highlighted the involvement of melatonin in the metabolism of ROS and upregulation of antioxidants, which are responsible for the stress resistance properties of melatonin in plants (Zhang, Sun, et al.,2015). In recent years, several important reports have improved our understanding of the mechanism of melatonin-mediated stress responses in plants. The first melatonin receptor candidate γ-protein-coupled receptor 2/phytomelatonin receptor 1 (CAND2/PMTR1) was identified in A. thaliana, where it was associated with receptor-mediated closure of stomata. It acts via heteromeric G protein α subunit-regulated H2O2 and Ca2+ signals. CAND2 receptor is located on the plasma membrane with seven transmembrane helixes that directly interact with melatonin. Their interaction triggers the dissociation of G protein α, β, and γ subunits, which in turn activates NADPH oxidase-dependent H2O2 production. The production of H2O2, intervened by the cell-membrane-located NADPH oxidase, activates Ca2+ channels and enhances the Ca2+ influx, resulting in inactivated inward K+ currents which facilitate stomatal closure (Wei et al.,2018; Wen et al.,2016).
In comparison to other phytohormones, melatonin has a secondary role as a signaling molecule, especially under stress conditions. Transcription factors play a crucial role in stress responses by directly regulating the transcription of stress-responsive genes, and by establishing a cross-talk between multiple signaling pathways (Arnao & Hernández-Ruiz,2015; Shi et al.,2015; Zhang, Sun, et al.,2015). In A. thaliana, the melatonin-mediated stress response involves four transcription factors, i.e., Zinc Finger protein 6 (ZAT6), which is essential for melatonin-mediated freezing stress response, Auxin Resistant 3 (AXR3)/IAA inducible 17, which contributes to the process of natural leaf senescence, class A1 Heat Shock Factors, which are involved in melatonin-mediated thermotolerance, and C-repeat-Binding Factors (CBFs)/Drought Response Element Binding 1 factors (DREB1s), which are essential for sugar accumulation and may also be partially involved in melatonin-mediated stress response. Moreover, diurnal changes in AtCBF/DREB1s expression may be regulated by corresponding changes in endogenous melatonin level, which could subsequently be involved in the diurnal cycle of plant immunity (Shi et al.,2016). Melatonin treatment also improves the level of sugars and glycerol in A. thaliana, leading to an increase in endogenous nitric oxide (NO), which confers immunity against bacterial pathogens via SA and NO-dependent pathways (Qian et al.,2015; Shi et al.,2016). Furthermore, Lee and Back (2016) reported that mitogen-activated protein kinase (MAPK) signaling, through MAPK kinase 4/5/7/9-MPK3/6 cascades, is also required for the melatonin-mediated establishment of innate immunity in plants.
Conclusion
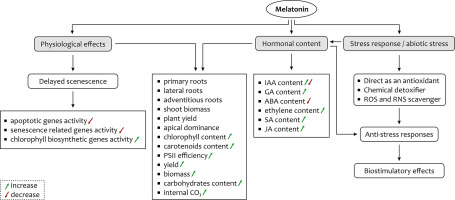
The data presented here prove that melatonin is a potential signaling molecule with a substantial impact on the maintenance of plant growth and development and mitigation of various abiotic stresses (Figure 2). It plays an important role in overcoming stress in plants by maintaining the germination process as well as growth and development by increasing the GA level. It improves the rate of photosynthesis by increasing the level of associated proteins, efficient CO2 assimilation, and by protecting the cell against damage induced by adverse effects of stress. In addition, the role of melatonin in altering the activity of senescence-associated genes and alleviating the level of various phytohormones, and its ubiquity among different plant species may indicate its potential application for improving yield in a wide variety of crops. It is evident that impressive advances have been made in the field of melatonin research in plants, relating not only to its universal presence but also to its role in different physiological functions. However, many questions related to melatonin remain to be answered.
Handling Editor
Robert Konieczny; Jagiellonian University, Poland; https://orcid.org/0000-0002-4205-2656