. Introduction
All living organisms react to changes, and they must adapt to the current conditions through morphological and genetic alterations in order to survive. Phenotypic plasticity is especially important for plants because it is a general adaptation strategy to variable, often stressful factors and is an equivalent of the animal’s defense mechanism, i.e., escape. Many plant species have the ability to modify physiological and developmental processes, enabling adaptation to the current environmental conditions. The growth and changes of root system architecture (RSA) are an excellent example of such developmental plasticity, i.e., genes expression, signal transduction in roots, can be modified by minerals, water availability, and even light or wounding, resulting in different phenotypes. According to classic definitions, the root is an underground axial organ, which is part of the sporophyte that attaches the plant to the ground and provides it with water and nutrients. In addition, the root exhibits apical growth, positive gravitropism, and is a synthesis site of important growth regulators (i.e., cytokinins, auxins) and secondary metabolites (flavonoids) (Lynch & Brown, 2012; Montiel et al., 2004). In higher plants, three main root types are distinguished in organogenesis: main (primary) root, lateral roots, and adventitious roots (Fitter et al., 1991). Considering the types of root branching, we can distinguish a taproot system with one well-developed main (primary) root and numerous smaller lateral roots, which is characteristic of dicotyledonous plants, and a fibrous root system, with numerous lateral and adventitious roots that occurs in monocotyledonous plants (Atkinson et al., 2014). However, Arabidopsis thaliana can also form specific adventitious roots (AR) arising from the initials of the cambium, which are converted in the founder cells in response to wounding and the initiation of AR primordium is promoted by auxins and expression of WOX11 (Wuschel-related Homeobox 11) together with other transcription factor PRE3/ATBS1/bHLH135/TMO7 (Baesso et al., 2018; Ge et al., 2019). Adventitious roots can also form in dicots (i.e., tomato) in response to abiotic stresses such as stem wounding or cutting. It has been suggested that auxin protein transporters (LAX1, PIN3, PIN4, PIN7) may play a crucial role in delivering auxin to AR induction and initiation sites. In addition to auxins, ABA, zeatin and salicylic acid may also play a role in the induction, initiation, and emergence of the developing AR in tomato (Guan et al., 2019).
In the biological aspect, the term “architecture” of the root refers to the shape and spatial configuration (3D) of the root system (de Dorlodot et al., 2007; Satbhai et al., 2015). On a macro-scale, the architecture of the root system concerns such parameters as the length of the main root, the length and number of lateral roots, as well as the angle of their branching. On a micro-scale, it includes root diameter and root hair length and density analysis (Lynch, 1995). Depending on the physical and chemical properties of the soil, root system architecture is subjected to various modifications, consisting of the promotion or inhibition of the elongation growth of main roots, development of lateral roots, the proliferation of root hairs, and formation of adventitious roots (Motte & Beeckman, 2019; Osmont et al., 2007). Root system architecture determines the acquisition of minerals and the strategies of their uptake by the plant and is therefore a fundamental determinant of productivity. For this reason, much attention is currently being paid to research on the genetic and hormonal regulation of root growth and development as well as exogenous factors affecting root architecture. The progress that has been made over the past ten years in the application of modern techniques of molecular biology and genetics has allowed manipulation of root architecture towards its optimization and more efficient uptake of minerals and water to increase tolerance to various abiotic stresses, especially in crops such as rice, maize, potatoes, cassava or cereals (Khan et al., 2016; Koevoets et al., 2016; Meng et al., 2019). In recent years, great progress has been made in understanding molecular mechanisms controlling the development of the root system and regulating root growth by phytohormones in response to various environmental conditions (Meng et al., 2019; Scheres & Krizek, 2018). Currently, not only are visualization techniques being improved, which allow for live 3-D and even 4-D root observations to accurately depict the morphology, geometry and topology of the root system, but also reactions of single genes and whole quantitative trait locus (QTLs) in roots exposed to biotic and abiotic stresses are being examined (reviewed in Wachsman et al., 2015). Increasingly frequently, the current subject of research on RSA includes the creation of phenotypic models that are to reflect the structure of roots in response to any combination of deficiency or excess of minerals. The latest proposed workflow for in situ quantitative root biology allows to study of nuclear structure, cell cycle, cell and organ geometry analysis, and protein localization in the RAM, in the differentiation zone of the root, and lateral roots at single-cell resolution. These four modules for cellular phenotyping, especially I and IV are very useful to study lateral or adventitious root initiation that is dependent on local gene expression (Pasternak & Pérez-Pérez, 2021).
. Root responses to environmental clues
Mineral nutrients
Abiotic stresses resulting from global climate change, water scarcity, and mineral constraints dramatically reduce plant growth and yield. Changing the root architecture to improve the efficiency of water and nutrient uptake is one method to minimize the negative consequences of these factors. The formation of lateral root primordia and their growth is the main factor determining the architecture and size of the root system in dicotyledonous plants, which determines the effective adaptation to spatial and temporal changes in the availability of minerals (Hermans et al., 2006; Hodge, 2004).
Plants require 17 essential elements for the completion of their life cycle, namely C, H, O, Ca, K, Mg, N, S, P, Cl, B, Cu, Fe, Mn, Mo, Ni, and Zn. In situations in which a nutrient is not present in sufficient amounts to support its functional roles, it will lead to a state of deficiency and physiological disorders. In contrast, if the nutrient is in excess of the optimum, toxicity effects may occur. Therefore, not only nutrient deficiencies but also excesses are considered mineral nutrient stress in plants (Pandey et al., 2021).
Root architecture changes particularly quickly in response to alterations in the availability of nitrogen, phosphorus, and iron (Figure 1), and it is mainly these three elements that focus the most attention in the scientific literature.
Figure 1
Schematic presentation of root system responses to different nutrient supplies. The roots were grown in sufficient, low and high concentration of N, P, Fe exemplified by Arabidopsis thaliana Col 0. (A) nitrate, (B) phosphate, (C) iron.
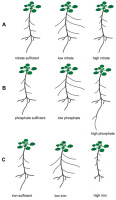
Initial physiological responses to the local availability of nitrates in soil include rapid induction of systems that transport these ions. Genes encoding nitrate transporters are then expressed more intensively in those parts of the roots that are directly exposed to nitrates (Forde & Walch-Liu, 2009). In addition, in terms of the root system, adaptation strategies that allow increasing in the uptake and transport of minerals from the soil include morphological changes, such as main root growth, lateral root formation and elongation, and root hair formation (Forde & Walch-Liu, 2009; Hodge, 2004). Particularly noticeable changes in the architecture of the root system are observed under different nitrogen conditions (Figure 1A) (Hermans et al., 2006; Malamy & Ryan, 2001; Shahzad & Amtmann, 2017). Under natural conditions, plants are often exposed to nitrogen deficiency, which is usually in deficit quantities in the soil. It is known that nitrates are the main source of nitrogen for plants growing in well-oxygenated soils. In addition to the structural function of nitrogen (a component of proteins, nucleotides, and nucleic acids), many scientific reports from recent years have confirmed the signaling role of nitrates, which can modify plant metabolism in response to the variable availability of these ions in the environment. Microarray data analysis revealed that the expression of about 40 genes in the A. thaliana genome is induced by nitrates (Forde, 2002). In addition, nitrate ions have been shown to be an important regulator of developmental processes, and one of the best examples of such regulation is the stimulation of lateral root development in many plant species, including A. thaliana, in response to local nitrate supplementation (Zhang & Forde, 2000). Classical experiments on the influence of mineral nutrition on the branching of the root system were already carried out in 1973-1978 on barley. It was shown in these pioneering experiments that local supplementation of high NO3− and NH4+ concentrations and inorganic phosphate (Pi) stimulated both the formation and elongation of lateral roots (Drew & Saker, 1978). More than twenty years later, research on Arabidopsis has shown that there is an increase in the number of lateral roots in response to local nitrate treatment (Forde & Zhang, 1998). Subsequent work on the effects of nitrogen on the growth and development of Arabidopsis roots has characterized the four main responses of these organs to different availability of this element. The stimulating effect of low nitrate concentrations (≤1 mM) on lateral root growth (Forde & Zhang, 1998; Zhang & Forde, 2000) and the general inhibitory effect of high concentrations of these ions (≥10 mM) on the activity of apical meristem of the lateral root has been demonstrated in Arabidopsis (Remans et al., 2006; Zhang et al., 2007). It is known that not only the ion concentration influences the RSA, but also the ions ratio plays a significant role in shaping the roots. It has been found that inhibition of lateral root primordium initiation occurs under the high C:N ratio (Malamy & Ryan, 2001). A higher accumulation of chloride (Cl−) leads to lower NO3− content in plants. This antagonistic interaction between Cl− and nitrate is one of the reasons why a high amount of Cl− is considered harmful to agriculture (reviewed by Xu et al., 2000).
The use of an alternative, organic nitrogen source in the form of L-glutamate induced rapid bud formation and development of lateral roots in Arabidopsis (Forde & Walch-Liu, 2009; Walch-Liu et al., 2006). An experiment confirming the signaling role of NO3− in controlling root development was carried out in tobacco mutants with impaired nitrate reductase gene that accumulated high amounts of nitrates in the tissues, and at the same time strongly inhibited lateral root growth (Zhang & Forde, 2000). Thanks to the availability of mutants and transgenic lines with disrupted nitrate assimilation pathway, it was also possible to detect developmental processes in Arabidopsis, in which nitrates act as signaling molecules. When Arabidopsis seedlings grew on a low nitrate concentration, a 2–3-fold increase in lateral root elongation rate was observed. The signal that led to intense divisions in the lateral root meristematic zone was directly associated with the presence of NO3− and not the assimilation products of these ions (Forde, 2002). Several genes have been identified that code potential elements of the signal transduction pathway, whose final effect is the emergence and development of lateral roots. Based on the conducted research, it is believed that transporter proteins, AtNRT1.1 and AtNRT2.1, also called transceptors, are “sensors” of the availability of nitrates in the environment in Arabidopsis (Gojon et al., 2011). The AtNRT1.1 protein is a transporter with different affinity for nitrates (low or high) depending on the amount of nitrogen available in the soil, and the AtNRT2.1 protein is a transporter with high nitrate affinity (Ho et al., 2009; Remans et al., 2006; Walch-Liu & Forde, 2008). The AtNRT1.1 sensor, which activates the expression of the ANR1 gene encoding the transcription factor from the MADS family, functions in a high local nitrate concentration (Remans et al., 2006). The constitutive expression of the ANR1 gene in the presence of nitrates strongly stimulates the growth of lateral roots but does not affect the length of the primary root (Desnos, 2008). Mutants of Arabidopsis thaliana with reduced levels of ANR1 protein had shorter lateral roots, and expression of the ANR1 gene in wild-type plants was induced by nitrate starvation (López-Bucio et al., 2003). In turn, the AtNTR2.1 transporter, with a high affinity for NO3−, was activated in plants with suddenly changed nitrate availability from high to low concentration (Remans et al., 2006; Vidal & Gutiérrez, 2008). Walch-Liu et al. hypothesized in 2006 the potential role of auxins in signaling, in response to the presence of nitrates in the medium. It has been proposed that the accumulation of large quantities of these anions in aboveground tissues generates a long-distance signal that controls lateral root development. It appeared that the transfer of Arabidopsis plants for 24 h from 50 mM to 1 mM nitrate medium caused a 50% increase in the content of indolyl-3-acetic acid (IAA) compared to plants grown only on 50 mM nitrates (Walch-Liu et al., 2006). These results suggest that a high concentration of nitrates in aboveground tissues inhibits the transport and/or biosynthesis of auxins in the roots, which results in the inhibition of lateral root development. Similar results were obtained in experiments carried out on soybean when the transfer of plants from 8 mM to 1 mM nitrate medium caused a 4-fold increase in the amount of IAA in the roots (Walch-Liu et al., 2005). Changes in auxin accumulation, caused by a high concentration of nitrates in the environment, might indicate an inhibitory role of these phytohormones in the lateral root formation. An interesting hypothesis has been proposed in 2010, which assumes that NTR1.1 is not only involved in the transport of nitrates but also of auxins. Under low nitrate concentration conditions, NTR1.1 participates in the basipetal transport of IAA from the lateral root apices and thus inhibits lateral root elongation (Krouk et al., 2010). Rapid progress has been made to dissect gene regulatory and hormonal networks during lateral root formation in dicotyledonous model plants such as Arabidopsis. Du and Scheres (2018) discuss how the auxins and auxin signaling control lateral root development in four chronological steps of lateral root formation (positioning, initiation, outgrowth, and emergence) focusing on transcriptional regulators involved in this process. Furthermore, with the rapid development of functional genomics and root type-specific QLTs analyses, significant advances have been made in elucidating the molecular regulatory mechanism of root development (primary root, crown root, lateral root, and root hairs) in monocot rice plant (reviewed by Meng et al., 2019).
It has been reported that primary and lateral root elongation in response to nitrogen deficiency depends on BRs synthesis and signaling. DWF1 as encoding a C-24 reductase that catalyzes the conversion of 24-methylenecholesterol to campesterol and (6-deoxo) dolichosterone to (6-deoxo) castasterone in the BR biosynthesis pathway. The total root length of dwf1 mutant (defective in BRs biosynthesis) was inhibited under mild- N deficiency. These results indicate that low nitrogen stimulates root growth via the upregulation of BR-biosynthesis genes (Jia et al., 2021). Previous studies revealed that low N induces primary root elongation via brassinosteroid signaling. It was proposed that mild N- deficiency upregulates the expression of co-receptor BAK1 and activates the BR signaling cascade which leads to root elongation (Jia et al., 2019). It has also been documented that expression of TAR2 (Tryptophan Aminotransferase Related 2), as a key enzyme that produces IAA in roots, is significantly increased under low nitrate conditions, resulting in a higher level of IAA in the developing lateral roots. The tar2-c mutant exhibits a shorter total lateral root length and reduced numbers of LRs (Ma et al., 2014). Moreover, several studies have provided evidence for the complex crosstalk of auxins (IAA) with Brassinosteroids (BRs) in regulating lateral root growth under low nitrogen conditions. Brassinosteroids are required for lateral primordia initiation while auxins affect the formation and development of lateral roots. BRs increase lateral root initiation by promoting auxin transport (Bao et al., 2014). However, a higher concentration of BRs inhibits LR formation via impending auxin signaling through auxin response genes AUX3/IAA17 (Kim et al., 2006). A very recent report shows that local auxin biosynthesis induces lateral root elongation while allelic coding variants of YUCCA8 determine the extent of elongation under N deficiency. By up-regulating the expression of YUCCA8/3/5/7 and of Tryptophan Aminotransferase of Arabidopsis 1 (TAA1) under mild N deficiency local auxin accumulation increases in LR tips (Jia et al., 2021).
Tan and co-workers (2020) demonstrated that exogenous application of salicylic acid (SA) inhibits primary root elongation and organogenesis of lateral roots by changing the phosphorylation status and thus localization and activity of PIN proteins which are responsible for the polar transport of auxins.
The research suggests that the plasma membrane proton pump – H+-ATPase is an important element involved in the regulation of root growth in response to different nitrogen and phosphate nutrition. The conducted experiments show that one of the 11 isoforms of this protein found in the Arabidopsis, namely AHA2, is involved in the regulation of root growth and development under different nitrogen nutrition. The aha2mutant had a much shorter main and lateral root, compared to the wild type, both in plants grown on a mineral (NO3−) and organic (glycine) nitrogen sources (Młodzińska et al., 2015). The new results suggest that under low phosphate concentrations, AHA2 acts mainly to modulate primary root elongation by mediating H+ efflux in the root elongation zone, whereas AHA7 plays an important role in root hair formation (Hoffmann et al., 2019).
In addition to nitrogen, phosphorus is one of the macroelements conditioning the proper growth and development of plants. Unlike nitrogen, it is quite difficult to access due to its low concentration (1 µM on average), poor solubility, and low mobility in soil solution (Lambers et al., 2006; Nussaume et al., 2011; Raghothama, 1999). In addition, about 90% of phosphorus in soil is present in organic form, and inaccessible to plants (Lambers et al., 2006). Another factor limiting the availability of phosphorus for plants is non-renewable and rapidly decreasing phosphorites, which are a natural source of phosphorus to produce phosphate fertilizers. Modifications of the root system architecture are among the adaptations that allow the plant to absorb phosphorus more efficiently. A number of literature data indicate that the availability of phosphorus determines the size and type of the root system of many plant species, including maize, rice, bean, white lupine, and tomato (Niu et al., 2013). Based on the published data, it is known that one of the adaptation mechanisms to phosphorus deficiency is primary root growth inhibition, strong growth and development of lateral roots, and increased proliferation and length of root hair (Figure 1B and Figure 2) (Kawa et al., 2016; Niu et al., 2013; Péret et al., 2011; Williamson et al., 2001). It should be mentioned that in Arabidopsis thaliana only T cells of epidermis in the maturation zone differentiate into root hair (Pasternak et al., 2022).
Figure 2
Arabidopsis root hairs morphology is affected by Pi concentration. Root hairs were photographed with Olympus S2X9 DP 71 Olympus, Japan, Cell^B - Image-acquisition and archiving Software Olympus.20x. (A) sufficient level of Pi, (B) low level of Pi.
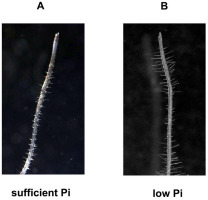
However, at high phosphorus concentrations, inhibition of lateral root growth and primary root elongation were observed (Linkohr et al., 2002). Specific changes in the root architecture caused by low phosphate concentrations, which occur only in some plants, should also be mentioned. These are, e.g., the formation of atypical roots, root clusters in non-mycorrhizal plants, or proteoid roots in the Proteaceae family (Watt & Evans, 1999). Cluster roots are a large number of determinate branch roots that develop on the main root axes. A detailed description of cluster root morphology and functioning in white lupin and different species of Proteaceae was published in 2011 (review by Lambers et al., 2011).
Gene groups (QTL) have been identified that are responsible for changes in root structure and morphology in Pi deficiency conditions. The LPR1, 2, 3 genes (low phosphate root 1, 2, 3), which are expressed in the cap cells and root apex, encode copper oxidases, which in turn modify the activity and transport of hormones in Pi deficiency, which results in main root elongation growth inhibition and induction of lateral root development (Svistoonoff et al., 2007). Under low Pi availability, the PDR2 gene (phosphate deficiency response 2), encoding type 5 ATPase, participates in the regulation of cell division activity in the root apical meristem (Ticconi et al., 2004). It is also believed that the inhibition of root growth in plants exposed to phosphorus deficiency is associated with the toxic effect of over-optimal iron accumulation (Svistoonoff et al., 2007). The summary of signaling components regulating root developmental responses to nitrate and phosphate deficiency was discussed in detail by Shahzad and Amtmann (2017).
Iron content in plants is relatively high compared to other microelements. However, excess iron is toxic and leads to the inhibition of primary root growth and lateral root development. This modification aims to limit the uptake of this element by the root (Li et al., 2016). Low iron availability causes morphological changes in the root epidermis, similar to changes caused by low phosphorus availability (Figure 1C). When iron availability is limited, a strong proliferation of root hair occurs (Li et al., 2016; Schmidt et al., 2000). The POPEYE protein (PYE) is one of the transcription factors that participate in the regulation of root growth under Fe deficit conditions. A mutation of the gene encoding this protein causes severe disorders in lateral root development, and also limits the elongation growth of the main roots, and induces leaf chlorosis in conditions of iron deficiency (Long et al., 2010). Recent studies have revealed that one of the transcription factors (bHLH115) in A. thaliana regulates the expression of genes associated with the maintenance of normal Fe homeostasis during this microelement deficiency. It has been proved using immunoprecipitation that bHLH115 binds to promoters of such genes as bHLH 38, 39, 100, 101, and POPEYE. A mutation in the bHLH115 gene (bhlh115 mutant) causes a greater sensitivity of the plant to the lack of iron in the environment, and increased iron accumulation occurs when it is overexpressed. The bhlh115 mutant has shorter roots and significantly lower chlorophyll content compared to the wild type (Liang et al., 2017). A better understanding of the root genetics (from gene to function) is needed to design root system with enhanced soil exploration and nutrient uptake in low soil fertility.
Water
Water is an important factor, whose deficiency, in addition to minerals, dramatically reduces plant growth. It is well known that water stress mainly refers to the lack of water in the soil. Under these conditions, plants cannot absorb water and even lose it, which causes osmotic stress in the cells. The commonly known reaction of plants to drought described by many researchers is a strong root elongation growth, enabling the use of groundwater resources at great depths (Comas et al., 2013). This adaptation is characteristic of many desert plant species that have deep taproots and shallow lateral roots (Lynch, 1995). However, only in 2014, Bao et al. described the phenomenon of sensing water in the environment (hydropatting), a complex plant response that determines the location of lateral roots, root hair formation, and aerenchyma differentiation. In Arabidopsis thaliana hydropatting is dependent on SUMO-mediated posttranslational regulation of auxin signaling pathway (ARF7/IAA3) controlling lateral root branching pattern in response to water availability (Orosa-Puente et al., 2018).
It has also been shown that plants are able to partially inhibit gravitropism and reprogram the root system so that it grows towards higher humidity, so-called root hydrotropism (Eapen et al., 2005). Research on Arabidopsis, maize, cucumber, and pea has revealed that the main root changes the direction of growth in response to low water potential (Mizuno et al., 2002; Takahashi & Scott, 1991; Takahashi et al., 2002). It has been observed in Arabidopsis thaliana that the development of lateral root buds and thus the development of these organs is inhibited under osmotic stress conditions (Figure 3), and abscisic acid (ABA) is involved in this reaction. Under osmotic stress conditions, lateral root length was significantly greater in the aba2-1 and aba3-2 mutants with reduced ABA levels compared to wild-type roots, suggesting that ABA is an important regulatory element that inhibits lateral root growth in response to osmotic stress (Deak & Malamy, 2005). To date, only some genes related to root response to drought have been identified. Increased activity of the (Plasma membrane Intrinsic Protein) PIP1:1, PIP1:5, and PIP2:4 genes have been observed in maize roots in response to osmotic stress. These genes encode plasma membrane channel proteins from the aquaporin family, responsible for water transport that can prevent root water loss (Kudoyarova et al., 2015). A. thaliana edt1 mutant (extremely drought tolerant 1) is characterized phenotypically by very long roots. The EDT1 gene has been shown to encode the HD-ZIP transcription factor (HDG11), which directly activates the expression of genes encoding proteins involved in cell wall relaxation, and these, in turn, promote root elongation (Yu et al., 2014). In addition, quantitative trait loci (QTL) markers that control the angle of root bending in rice have been successfully identified. High expression of the DRO1 (deep rooting 1) gene causes strong downward bending of the roots. The introduction of this gene into plants with a shallow root system caused an increase in drought tolerance and a significant root system growth to a greater depth (Uga et al., 2013). A recent study has identified several genes in rice related to root system architecture that confer a yield advantage during conditions of drought i.e., HVA1 encoding late embryogenesis abundant (LEA) protein. HVA1 was highly accumulated in root apical meristem and lateral root primordia after ABA and stress treatments, leading to enhance the expansion of the root system (Chen et al., 2015). Soil flooding is another environmental stress factor that can impair root growth, as a result of hypoxia. Soil is filled by water and gas diffusion is greatly reduced. The root system is now becoming a focus of research at low oxygen conditions. It has been described that the primary root of Arabidopsis thaliana grows sidewise in reduced oxygen surroundings. This root bending is inhibited by the activity of the VII ethylene response factor (RAP2.12) under hypoxia. Authors suggested that restriction of root bending is induced by altered auxin signaling at the root apex because RAP2.12 promotes the accumulation of polar auxin transporter PIN2 (Eysholdt-Derzsó & Sauter, 2017). Moreover, recent studies on rice revealed that activation of OsPIN2 in epidermal cells might regulate adventitious root emergence under flooding. The formation of adventitious roots is a characteristic response to flooding. The growth of adventitious roots and repression of lateral root development by ERFVIIs under low O2 conditions change the root system architecture with a shift from underground to aerial roots. These roots are located closer to the shoot thereby facilitating oxygen supply in flooding-tolerant and intolerant plants (Lin & Sauter, 2019; Shukla et al., 2019). A recent minireview highlights the molecular mechanisms involved in the spatial distribution of lateral roots of maize and their branching in response to the availability of nutrients (nitrogen and phosphorus) and water (Yu et al., 2019). Despite extensive research, there is still little data on the molecular mechanisms that control root architecture in response to a lack or excess of water in the soil. The recent findings on the root anatomical and morphological traits to plant adaptation to waterlogged soils with low oxygen status have been reviewed by Pedersen et al. (2021). The authors demonstrate the models of the molecular mechanism of constitutive aerenchyma formation in roots, lateral root growth, and adventitious root development with a large cortex – to stele ratio which is involved in the acclimation of rice to soil waterlogging. It has also been suggested that these responses are regulated by auxin transport and signaling (Pedersen et al., 2021).
Figure 3
Schematic presentation of Arabidopsis thaliana root system responses to different abiotic stress factors. (A) optimal conditions, (B) low water stress, (C) salinity, (D) low temperature, (E) UV-B radiation, (F) vanadium.
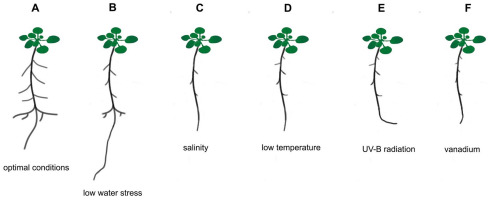
The newest study on two contrasting varieties of rice, a drought-tolerant ‘Heena’ and a sensitive ‘Kiran’ revealed that the ‘Heena’ variety can better defend against drought stress by inducing numerous abiotic stress-responsive genes (LEA – Late Embryogenesis Abudant, DREB – Dehydratation Responsive Elements), transcriptional factors that regulate the expression of many stress-inducible genes (AP2/ERF, MYB, WYRKY, bHLH) and genes involved in antioxidative mechanisms and photosynthesis. The root morphology and anatomy were also altered in ‘Heena’ rice compared to ‘Kiran’ (the number and length of seminal roots were higher than adventitious roots, the number of adventitious roots was significantly reduced, and increase in the aerenchyma formation, xylem cells, and lignification of sclerenchyma and endodermis was observed) to maintain membrane integrity and water content in plants under drought stress conditions (Tiwari et al., 2021).
Salinity
Salt stress affects the growth and productivity of plants as a result of two component interactions: ionic stress associated with an excessive concentration of Na+ ions, and osmotic stress caused by a decrease in soil water potential. Osmotic stress generates early plant responses, such as inhibiting cell growth and closing stomata to minimize water loss. In contrast, an increase in the content of Na+ ions in tissues leads to subsequent defense responses, consisting of a drastic reduction of sodium transport from the root to the above-ground tissues and storage of Na+ ions in vacuoles and stele cells in the root (Munns & Tester, 2008). Experiments were carried out on A. thaliana to determine which root system parameters are sensitive to salt stress. Plants were grown on agar plates with various NaCl concentrations (from 0.25 mM to 150 mM), and three root architecture parameters were characterized: root length, mean lateral root length, and number of lateral roots formed. The research was carried out on 32 A. thaliana lines and four strategies of root adaptations to salinity were identified based on the analyses. Strategy 1, characteristic, e.g., for the Columbia (Col 0) ecotype, was manifested by a stronger reduction in the growth of main roots compared to lateral roots and a smaller number of lateral roots. Strategy 2, salinity caused the same growth inhibition in all measured parameters, e.g., Vanouver 0 (Van 0). Strategy 3, the plants had more reduced lateral root growth compared to the main root, e.g., Brunn ecotype 0 (Br 0). Strategy 4, where the plants had strongly shortened main roots and strongly inhibited development of lateral roots, e.g., the JEA ecotype, Mr-0 (Julkowska et al., 2014). These results have confirmed earlier reports that main and lateral roots react differently to high salt concentrations in the soil (Duan et al., 2013). Main roots responded with lower growth inhibition compared to lateral roots under high NaCl concentration. The role of abscisic acid in regulating root growth under salinity has also been demonstrated in this work. Mutants with a restricted ABA biosynthesis pathway and a disrupted ABA signal transduction pathway were more resistant to salt stress and showed weaker inhibition of lateral root growth than the wild type. In addition, the ProRAB18::GFP gene, which is an ABA-induced reporter gene, was highly expressed after plant treatment with 100 mM ABA (Ding & De Smet, 2013; Duan et al., 2013). Later reports have revealed that an increase of ABA level during salt stress causes inhibition of the signal transduction pathway controlled by gibberellins, which leads to a decrease in meristematic activity in the lateral root apices, a decrease in the rate of cell divisions and thus growth inhibition of these organs (Julkowska & Testerink, 2015).
Light
Although light is a morphogenetic factor that initiates certain changes in the growth and development of aerial parts, the root also responds to this signal. All major photoreceptors, such as phytochrome, cryptochrome, and phototropin are present in the root, but the mechanism of light perception and signaling in the context of growth and developmental changes is still poorly understood (Galen et al., 2007). Recently, it has been demonstrated, thanks to the innovative method of root visualization using the “GLO-Roots” (growth and luminescence observatory for roots) that the root reacts to light (Rellán-Álvarez et al., 2015). It has been shown that the expression of genes encoding photoreceptors occurs at the root apex: in the root cap, meristematic and elongation zone (Mo et al., 2015). A. thaliana roots show a negative phototropic response to blue light mediated by phototropin 1 (PHOT1), while root phototropism is positive in response to red light, with the involvement of phytochrome A and B (PHY A and PHY B) (Kiss et al., 2003). In addition, the role of phytochrome A (PHY A) in the induction of root elongation after irradiation with blue light and far red has been confirmed (Correll & Kiss, 2005). Two transcription factors have been identified that regulate Arabidopsis root growth after exposure to light: COL3 (constant-like 3), inducing the formation of lateral root buds after irradiation with red light, and HY5 (basic region leucine zipper transcriptional factor), controlling the initiation of lateral root buds, elongation growth of main roots and root hair proliferation (Oyama et al., 1997). It was also observed that in the darkness, root growth of Arabidopsis WT plants was notably stimulated, while it was inhibited after light exposure. Light-mediated root growth suppression seems to be negatively regulated by a master regulator of photomorphogenesis HY5. The expression of HY5 is induced by light, in turn, it inhibits root growth (Zhang et al., 2019). Ultraviolet light is an integral part of solar light, and UV-B (280–320 nm) reaches the surface of the earth and affects plants and animals. The newest studies reported that UV-B inhibits the root growth of Arabidopsis seedlings by restraining cell division and elongation (Lyu et al., 2019). Recently, it has been reported that UV-B radiation, besides growth suppression, induces root bending toward the UV-B radiation. It was observed that UV-B significantly reduces total auxin accumulation in roots by repressing auxin biosynthesis in roots. However, it increases auxin distribution on the non-radiated side of the root tip and thus promoting root bending. The research also indicated the involvement of flavonoids in the auxin–dependent root bending in response to UV-B radiation (Wan et al., 2012). Moreover, several studies have also suggested the role of gibberellins (GAs) in UV-B root growth inhibition, because UV-B blocks the GAs synthesis or transport-related genes expression as well as induces the expression of GA2-oxidase gene and inactivates the GA (Lyu et al., 2019). The elevated UV-B radiation leads to an increase in the content of other phytohormones such as abscisic acid, jasmonic acid and these inhibit the cell division of root tip and in turn, suppress root growth (Zhang et al., 2019).
Temperature
Temperature is one of the abiotic factors that changes rapidly in the root zone depending on the time of day, season, and depth of the soil. Soil temperature strongly affects the root system and the uptake of water and nutrients (Walter et al., 2009). It is worth noting that different plant species differ significantly in the temperature range that is optimal for root development, e.g., the optimum for oat is 4–7 °C, 14–18 °C for wheat, 15–20 °C for peas, 22–25 °C for tomatoes, 25–30 °C for sunflowers, and 32–35 °C for cotton. The roots of mono- and dicotyledonous plants react similarly to temperature fluctuations by reducing the length of the main roots, reducing the number of emerging lateral root buds, and changing the inclination angle of the emerging lateral roots (Koevoets et al., 2016). Arabidopsis studies on low temperature stress (4 °C) have revealed that inhibition of the root elongation rate is associated with disturbances in basipetal auxin transport in the root through blocking the intracellular vesicular transport of PIN2 and PIN3 proteins facilitating auxin outflow from the cell. As a consequence, there is an accumulation of over-optimal auxin concentrations in root cells, and thus their growth inhibition (Shibasaki et al., 2009). Low temperature also reduces the rate of cell division in the apical meristem, which leads to a physical decrease in the surface and number of cells (Zhu et al., 2015). Studies on mutants with impaired AHP1-1 (ahp1-1), AHP2-1 (ahp2-1), and AHP3 (ahp3) genes, which are involved in cytokinin “signaling”, i.e., phytohormones inducing cell division, confirmed their involvement in root system growth regulation in response to low temperature (Zhu et al., 2015).
Few studies have been conducted on the effects of high temperature (40 °C) on the root system. It is generally accepted that root growth inhibition at high temperatures is associated with auxin transport modification and increasing ethylene levels in the roots (Qin et al., 2007).
Heavy metals and hazardous contaminations
Soil contamination with heavy metals and toxic substances from industrial activities can have diverse effects on the root system architecture. In Arabidopsis thaliana the exposure to common pollutants such as cadmium (Cd) caused a significant decrease in primary and lateral root length (Sofo et al., 2017). Cd inhibits primary root growth by reducing the size of the apical meristem due to a decrease in the cell number of the quiescent center (QC) (Bruno et al., 2017). By contrast, arsenic (As) increased the primary root length, and both Cd and As induced lateral root density. It has been proposed that Cd and As affect the auxin metabolism and distribution in the lateral and adventitious root apices (Piacentini et al., 2020). Recently, a detailed overview of the crosstalk between the activity of several hormones (auxins, jasmonates, ethylene, brassinosteroids) and the development of the root system of Arabidopsis in heavy metals stress was published (Betti et al., 2021). This summary highlights the important role of NO in root growth responses to Cd and As and pointing the interactions between NO and phytohormones (Betti et al., 2021). Sofo et al. (2022) analyzed the root structure and morphology of Arabidopsis in response to six heavy metals (Hg, Cd, Pb, Cu, Zn, and Ni) at the minimum toxic concentrations (from 2 µM HgCl2 to 550 µM ZnCl2). The results showed that Pb, Cu, Ni, and Zn induced main root length compared to control plants, and except for Ni and Hg, stimulated the development and growth of lateral roots. The observed modifications of root system architecture could be related to defense mechanism and root adaptation during harmful pollution in soil. Numerous studies on various crops demonstrated that toxic levels of vanadium (18–510 mg/kg) in the soil lead to root growth inhibition (reviewed in Chen et al., 2021). This decrease in root length and root biomass of crops can be attributed to the vanadium-dependent overaccumulation of ROS and in consequence membrane lipid peroxidation, inhibition of H+-ATPases, reduction of cell elongation, and mitotic cell division, as well as an antagonistic effect on uptake of essential macronutrients i.e., P, Mg, Ca (Aihemaiti et al., 2020; Chen et al., 2021). The interesting study monitored the influence of diclofenac (DCF) on the developmental parameters of the root system. DCF (2-2-2, 6-dichlorophenyl amino-phenyl acetic acid) is a pharmaceutically active anti-inflammatory compound frequently detected in worldwide soils (Acuña et al., 2015). The results showed that DCF inhibited the growth of Arabidopsis main root and emergency of lateral roots and caused oxidative stress (overproduction of H2O2) in the root tissues (Cho & Kim, 2021). In contrast, the elongation of lateral roots was stimulated when the concentration of DCF was increased. The authors suggested that DCF altered the root system architecture in a similar manner to auxins, but with lower activity. Probably, DCF suppresses the IAA signaling in roots (Cho & Kim, 2021).
. Root hair and abiotic stresses
Dynamic modification of root hair growth, their length, and density facilitate the absorption of water and nutrients from soils. The growth and development of plant root hairs include cell fate determination, root hair initiation, and elongation (Bibikova & Gilroy, 2002). The genes involved in epidermal cell fate determination include TRANSPARENT TESTA GLABRA (TTG), GLABRA3 (GL3), ENHANCED OF GLABRA (EGL3), WAREWOLF (WER), GLABRA2 (GL2), CAPRICE (CPC), TRIPTYCHON (TRY) and ENHANCER OF TRY and CPC (Li et al., 2022). Phytohormones play important regulatory roles as signal molecules in the growth and development of root hair. Some phytohormones, e.g., auxins, ethylene, and cytokinins promote root hair growth, while brassinosteroids and ABA suppress it, by regulating transcription of root hair-associated genes (WER, WGL2, WCPC, and HAIR DEFECTIVE6 (WRHD6)) (Vissenberg et al., 2020). Other hormones such as JA and strigalactones may regulate root hair growth through their crosstalk with the auxin/ethylene signaling pathways (Lee & Cho, 2013; Li et al., 2022). Root hairs are also affected by environmental stressors such as drought, salinity, and nutrient depletion. Under osmotic stress, root hair development is reduced, and the transcription factor GL2 negatively regulates root hair growth (Wang et al., 2020). Salinity inhibits root hair length and density by reducing polar auxin transport and auxin signaling (Liu et al., 2015). In low phosphate conditions, root hair cells grow larger and denser (Figure 2). It has been proposed that increased auxin level and signaling are involved in root hair modulation under Pi deficiency. In contrast, high Pi conditions inhibit root hair growth by repressing RSL4 expression (Vissenberg et al., 2020). Under Fe – deficiency plant roots increase their absorptive surface by inducing root hair branching and the auxin/ethylene signaling network is involved in this response (Vissenberg et al., 2020).
Current studies focus on the environmental and regulatory mechanism of phytohormones on root hair growth and development in crops e.g., maize (Vetterlein et al., 2022).
. Perspectives
All research on phenotypic traits and molecular regulatory mechanisms of phytohormones in the regulation of root growth and development is expected to lead in the future to crop plants with roots that will efficiently extract nutrients from poor soils and resist adverse soil conditions such as drought, salinity or nutrients deficiency. Great methodological advances in the molecular and genetic regulation of RSA to individual stress and a combination of stresses have been recently made. The next step is to manipulate these genetic pathways in order to enhance the tolerance of plants to environmental stresses. Moreover, very little is known about protein–protein interactions which might be responsible for modification of primary and lateral root growth in optimal and suboptimal conditions. Applying new approaches to study root developmental processes “in situ” will enable more reliable measurements of root traits and identify protein networks regulating root growth-related biology.


