. Introduction
The spread of invasive plants is intensifying due to ongoing climate change and disruption caused by human activities. It poses a threat to the functioning of ecosystems, for example, by changing the composition of plant species communities (Aerts et al., 2017; Castro-Díez et al., 2016; Kumar Rai & Singh, 2020) and reducing biodiversity (Aslani et al., 2019; Kumar Rai & Singh, 2020; Vilà et al., 2011). One of the invasive plant species found in European forests is black cherry (Prunus serotina Ehrh.) Black cherry has restricted natural succession and reduced seed germination, thereby inhibiting the natural regeneration of the forest in areas controlled by this species (Aerts et al., 2017; Halarewicz & Żołnierz, 2014); this effect extends to the related native common cherry (Prunus padus L.) Common cherry grows in fertile habitats, alder forests, mixed forests, and riparian forests; it can be found along streams and lake shores and prefers partially shaded locations. On the other hand, black cherry is moderately light-demanding but grows and reproduces most intensively in sunlit habitats (Closset-Kopp et al., 2011), most often in forests (Halarewicz, 2011). Unfortunately, black cherry is increasingly encroaching on the fertile habitats of alder forests and riparian forests (Dyderski & Jagodziński, 2015).
Alien tree species [e.g., P. serotina (Fruleux et al., 2023; Korzeniewicz et al., 2020), Pseudotsuga menziesii (Mirb.) Franco (Damszel et al., 2021), or Thuja plicata Donn ex D.Don (Baranowska et al., 2023; Damszel et al., 2021)] in Poland have been studied for many years; yet, it is still unclear why some alien species become invasive and others do not. The establishment of symbiotic relationships is considered an underlying reason, among others (Moyano et al., 2020). Most plants form mycorrhizae with fungi that increase the diversity, competitiveness, and productivity of ecosystems (Aslani et al., 2019). Introduced tree species can form mycorrhizae with both cosmopolitan taxa of fungi (occurring both in the natural range of the tree species and in the locations of its introduction) and alien taxa (Pringle et al., 2009). Menzel et al. (2017) showed that mycorrhizal associations were correlated with the geographic expansion of alien plant taxa, as alien species that formed mycorrhizae occupied a larger range than plants that did not form these associations. Moyano et al. (2020), studying the genus Pinus, found that the growth of invasive pine species was more dependent on ECM than that of other non-invasive species. Nevertheless, the role of mycorrhizal compounds in the mechanism of plant invasiveness remains incompletely understood (Aslani et al., 2019; Damszel et al., 2021). Little is known about the impact of alien species on soil microorganism communities (Lekberg et al., 2013).
Considering the threat posed by alien species, it is crucial to determine the relative importance of factors facilitating or inhibiting their invasion. This study aimed to compare the mycorrhizal frequency in the roots of closely related cherry trees, namely black cherry and common cherry, collected from three locations in Western Poland. The frequency of ectomycorrhizae was assumed to be higher in P. padus than in P. serotina. The frequency of ectomycorrhizae may vary depending on the location of the stands.
. Material and methods
Samples from thirty 2–3-year-old trees (15 P. serotina [Ps] and 15 P. padus [Pp]) were collected from three locations where the trees were adjacent to each other. The distance between the individuals of both species was at most 5 m (the pairs of P. padus and P. serotina bordered each other). Trees in every location were thoroughly dug out of the stands, and their entire root systems were selected for further research. Samples were collected in June 2022 from the buffer zone of the Dobrygość Forestry Nursery (Forest Experimental Station in Siemianice, division 13hx, habitat: fresh forest, 51.2499; 18.1648 [Dbg]), Złotówek (Oleśnica Śląska Forest Inspectorate, Grochowo Forestry, division 161d, habitat: fresh mixed forest, 51.3295; 17.2094 [Zł]), and Mrowino (Łopuchówko Forest District, Złotkowo Forest District, division 1h, habitat: fresh mixed forest, 52.5180; 16.7130 [Mro]).
The whole trees with roots were transported to the laboratory of the Department of Microbiology and Rhizosphere of the Institute of Horticulture of the National Research Institute in Skierniewice, where the tree root samples were assessed for the presence of mycorrhizal fungi. Ten grams of the roots of each tree were collected for further analysis. The roots were rinsed with a sieve with a mesh size of 2 mm. After transferring the roots to a Petri dish with distilled water, the mycorrhizal frequency was determined. The morphotypes of ectomycorrhizae were determined using the SMZ 800 NIKON stereoscopic microscope using the Colour Atlas of Ectomycorrhizae (Agerer, 1997; Hilszczańska, 2003). The most common morphotypes of ectomycorrhizae were photographed. The frequency was calculated using the following formula: 100 * number of mycorrhizal roots/total number of roots. For the description of the most common morphological types of mycorrhizae in the roots of Prunus seedlings, only well-developed mycorrhizae were considered (Hilszczańska, 2003). The average mycorrhizal frequency based on three root fragments was calculated. Fine roots from the upper, middle, and lower parts of the root system were cut into small fragments of approximately 1 cm (Parlade et al., 1996). After determining the mycorrhizal frequency, the roots were scanned using the EPSON EXPRESSION 10000 XL root scanner, and root growth characteristics were determined using WinRhizo software (Arsenault et al., 1995). The following root growth characteristics were calculated: root length, root surface area, root diameter, root volume, and number of fine root (tips).
The statistical analysis of the obtained results was performed using Statistica v.13 (StatSoft Polska). To analyze the significance of differences in the frequency of mycorrhizae (%), the Student’s t-test for independent samples was used. The observed values expressed as percentages were transformed according to the Bliss formula, where y = arcsin (p0.5). The impact of location on the frequency of mycorrhizae was assessed using the F test for a one-factor analysis of variance.
. Results
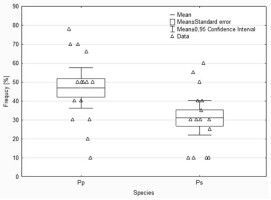
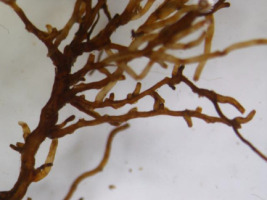
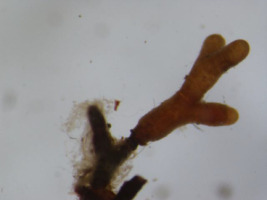
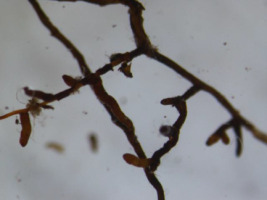
The analyses of the laboratory observations showed the dominance of morphotype 3 in all the studied roots of both common cherry and black cherry (light red to red muff, smooth light red to cream red, lighter tips of mycorrhizae; Table 1). A significantly higher frequency of mycorrhizae was observed in the roots of P. padus (p = 0.0213; α = 0.05). In this study, no impact of location on the frequency of mycorrhizae was observed (F test p = 0.1456; α = 0.05; Figure 1).
Table 1
Ectomycorrhiza type 3 on the roots of common cherry and black cherry in the studied locations.
| Prunus padus | Prunus serotina |
|---|---|
| Dobrygość | |
 |  |
 |  |
 |  |
 |  |
 |  |
| Złotówek | |
 |  |
 |  |
 |  |
 |  |
 |  |
| Mrowino | |
 |  |
 |  |
 |  |
 |  |
 |  |
The analysis of the morphological features of the cherry roots showed that in each of the studied locations, the tested roots of common cherry were longer than those of black cherry. The highest average root length (cm/plant) was found in common cherry collected from Złotówek (Pp_Zł) at 466.82 cm/plant and the lowest in black cherry (Ps_Dbg) at 273.56 cm/plant (Figure 2a). In each of the examined locations, the number of root tips (pieces/plant) of black cherry was lower than that of common cherry. The highest average number of root tips (pieces/plant) was observed in common cherry from Mrowino (Pp_Mro) at 972.6 pieces/plant, and the lowest number was found in black cherry from Dobrygość (Ps_Dbg) at 520.2 pieces/plant (Figure 2b). The average root area (cm2/plant), average root volume (cm3/plant), and root diameter (mm/plant) of black cherry in Złotówek were larger than those of common cherry. In other locations, the surface area and volume of roots were greater for common cherry. The largest average area was found in black cherry trees from Złotówek (Ps_Zł) at 95.08 cm2/plant and the smallest in black cherry trees from Dobrygość (Ps_Dbg) at 41.84 cm2/plant. Similarly, regarding root volume, the largest was found in black cherry from Złotówek (Ps_Zł) at 1.94 cm2/plant and the smallest in black cherry from Dobrygość (Ps_Dbg) at 0.54 cm2/plant. The largest root diameter (mm/plant) was found in black cherry from Złotówek (Ps_Zł) at 0.86 mm2/plant and the smallest in black cherry from Dobrygość (Ps_Dbg) at 0.5 mm2/plant. The highest proportion of type 3 mycorrhizae was found in common cherry in each location (Figure 2c–f).
Figure 2
Meaning: (a) length of tested roots (cm/plant); (b) number of root tips (pcs/plant); (c) surface area (cm2/plant); (d) root volume (cm3/plant); (e) root diameter (mm/plant) and (f) type 3 mycorrhiza frequency [%] P. padus (Pp) and P. serotina (Ps) from samples collected in Złotówek (Zł), Dobrygość (Dbg) and Mrowino (Mro).

. Discussion
Most trees and shrubs of the boreal and temperate zones form symbiotic relationships with ectomycorrhizal fungi under natural conditions. Usually, closely related trees and shrubs (belonging to the same genus) at this latitude tend to form similar ectomycorrhizal communities (Horton & Bruns, 1998; Ishida et al., 2007; Leski et al., 2010). So far, no studies have been conducted comparing the occurrence of mycorrhizae in black cherry, which is alien to forest ecosystems in Europa, with that of the native and closely related common cherry. However, the occurrence of mycorrhizae associated with P. serotina has been investigated. The average diameter of roots, the intensity of root branching, and the roots’ specific length were measured and compared, among other features, with those of common beech and Scots pine (Pinus sylvestris L.). Black cherry related mycorrhizal relationships similar to native tree species (Tardif et al., 2018). In our study, we showed that the frequency of ectomycorrhizae in common cherry was higher than that in black cherry. Nevertheless, it has been confirmed that black cherry can establish mycorrhizal relationships, including ectomycorrhizal ones, beyond the limits of P. serotina natural occurrence (Fruleux et al., 2023). Our research focused on assessing the frequency of ECM despite the common opinion that in the case of invasive species, arbuscular mycorrhizae, which are mainly responsible for the success of their expansion, should be expected (Heklau et al., 2022; Wilgan, 2020).
The formation of ectomycorrhizal networks by P. serotina in Europe’s temperate forests supports the “enhanced mutualism hypothesis”, which explains the success of this species’ invasion in this area (Fruleux et al., 2023). In addition, mycorrhizal fungi can provide protection against the pathogens of root systems (Arya et al., 2010), which increases their resistance and chances of survival. Mycorrhizae have been proven to either be indifferent to black cherry or positively affect its growth and development. A negative impact of symbiotic relationships on this tree species has not been indicated (Ibáñez & McCarthy-Neumann, 2015).
Like in our research, Tardif et al. (2018) found no significant differences between the root morphological features (root diameter or length) of black cherry, and another native tree species. However, the roots of black cherry from Dobrygość (DbG) were distinguished by smaller features, for example, length. This may be because these black cherry trees grow in the most fertile habitat. Although P. serotina spreads effectively across different habitats, it prefers less moist and less fertile soils (Dyderski & Jagodziński, 2015). Perhaps greater competition (varied species composition of the stand) and a potentially suboptimal habitat (fresh forest) for black cherry contributed to its smaller size.
The ability of trees to form mycorrhizae with many species of ECM is of great importance for many native and alien plant species in the process of spreading to and colonizing new areas (Woziwoda et al., 2012). The ability to create a symbiosis with native species of ECM is mentioned as one of the factors affecting the adaptation of Quercus rubra L. to local conditions beyond the limits of its natural range, its competitiveness in relation to native tree species, and its ability to survive and expand. Its adaptive success is enhanced by the establishment of ectomycorrhizal relationships with species of cosmopolitan fungi as well as with a broad spectrum of host plants (Reich et al., 2005; Trocha et al., 2012). The frequency of ectomycorrhizae in the roots of P. serotina indicates that the establishment of ectomycorrhizal relations could have had a positive impact on its expansion in European forests because it is recognized that mycorrhizal symbiosis may play an important role in the process of plant invasion (Pringle et al., 2009; Richardson et al., 2000). In further research, it will be important to identify the fungal species that form mycorrhizal relationships with black cherry and indicate whether these species are common to both bird cherry species, namely P. padus and P. serotina. This is important in the context of the prevailing belief that the elementary factor influencing the success of the primary colonization of new habitats is the formation of mycorrhizal associations, the inoculum of which may most likely come from fungi inhabiting forests in the new area of occurrence (Ashkannejhad & Horton, 2006; Jumpponen et al., 2002). In this study, we proved that black cherry could form ectomycorrhizae, which probably positively influenced the success of its invasion.
. Conclusions
We showed that the frequency of ectomycorrhizae in common cherry was higher than that in black cherry. Nevertheless, it has been confirmed that black cherry can establish mycorrhizal relationships, including ectomycorrhizal ones, beyond the limits of its natural occurrence. This ability probably positively influenced the success of its invasion. In further research, it is essential to identify the species of fungi that form mycorrhizal associations with black cherry and indicate whether these species are common to both cherry species.