. Introduction
Mushrooms, owing to their medicinal and food values, have been historically viewed as an amicable part of human society and have, at times, superseded plant and animal-procured products. Mushrooms are recognized as functional foods with nutritional value, structural quality, and acceptability (Greeshma et al., 2018; Mwangi et al., 2022; Pinto et al., 2020). Moreover, mushrooms undergo remarkable adjustments on imminent days for adaptation as smooth-running therapeutic foods. The consumption of mushrooms in Asian countries, such as India and Japan, is increasing at a significant rate. Based on data from Research and Markets (2021) (https://www.researchandmarkets.com), the value of the mushroom cultivation market was 16.7 billion $ (USD) in 2020 and is projected to reach 20.4 billion $ (USD) by 2025 (Landínez-Torres et al., 2021). Palatable mushrooms are low-calorie, low-fat food adjuncts with moderate amounts of proteins, carbohydrates, vitamins, minerals, amino acids, and dietary fiber (Johnsy et al., 2011). Mushrooms also exhibit antibacterial, antifungal, antiviral, antiparasitic, antioxidant, anti-inflammatory, antiproliferative, anticancer, anti-HIV, hypocholesterolemic, antidiabetic, and anticoagulant activities (Ghosh et al., 2020a, 2020b; Manimaran et al., 2021). Recently, Singh and Varshney (2020) estimated the food value of Astraeus hygrometricus, which was found to contain protein (16.02%), total carbohydrates (55.76%), fat (3.5%), monounsaturated oleic acid (4.59%), ash (3.8%), energy (159.5 kcal), pro-vitamin D2 (ergosterol) (1.09 mg/g), and dietary fiber (39.78%). Among the total free amino acids (8.20 mg/g), seven essential amino acids (3.9 mg/g), including tyrosine (0.98) and leucine (0.92), were found. These researchers also found a high amount of selenium in A. hygrometricus. In this work, we opted to focus on the mushroom Astraeus sp., owing to its food values of Astraeus sp. and its prominent use as a restorative drug owing to its antidiabetic, anticancer, hepatoprotective, anti-inflammatory, and cardioprotective activities (Phadannok et al., 2020). The primary aim of this study was to reveal the biodiversity of Astraeus asiaticus via morphological, biochemical, and molecular research in different woodlands or forests of the Bankura district, a red lateritic zone in West Bengal. Mycorrhizae provide nutrients between the roots of the host plant and fungi in the soil (Malajczuk et al., 1982). Biodiversity is a very important “natural capital.” Different varieties, species, genera and their complex interactions enable a functional ecosystem and productive economies (World Bank, 2022). Although wild mushrooms and fungi are an integral part of a given ecosystem, a study of their diversity and type has not been performed, particularly in tropical countries such as India. As a result, a knowledge gap exists. Wild edible mushrooms act as natural resources for rural people and provide nutrition (Meena et al., 2020). Accordingly, an investigation of the biodiversity of wild edible mushrooms and the consumption of this remarkable natural resource is of great economic importance. Astraeus species are economically important as they are collected by people for consumption and sale in commercial markets (Manna & Roy, 2014). Bankura is recognized for its great abundance, as residents can earn income by selling this wild edible mushroom. Thus, this mushroom is of great socioeconomic importance in the rural areas of West Bengal in India. A. asiaticus is a promising edible mushroom; however, its therapeutic value has not been explored. Therefore, in this study, the molecular characteristics, biodiversity, mycochemistry, antioxidant content, and antioxidant activity of this mushroom were determined to enable future research on its medicinal importance.
. Material and Methods
Survey of Habitat and Sample Collection
A survey on mushrooms was conducted in the Beliatore, Joypur, and Gangajal-ghati forest areas in Bankura. Joypur and Beliatore in the Bankura district are located in the southwest part of West Bengal and are associated with native extremities owing to the Damodar and Dwarakeswar rivers, which indicate the depletion of both forest range areas. The Damodar and Dwarakeswar rivers are almost parallel toward the southeast, as the general slope of the district runs from northwest to southeast. A periodic survey was conducted in the preferred area for the collection of A. asiaticus during the rainy season from mid-July to August 2019.
The habitat of mushrooms from the collection zone was noted, and photographs were captured. Soil samples from a rooting depth of 30 cm were collected from 15 zones (60 quadrats) of three forests. The samples were transported to the laboratory and processed for soil chemical parameter analysis following the standard method (Das, 2021; Iwabuchi et al., 1994).
Identification of the Mushroom Species
Mushroom (basidioma) morphology was examined macroscopically, and its size, shape, texture, color, and habit were determined, according to the pioneering work of Phosri et al. (2007). Biochemical tests were performed by dispensing a drop of chemicals (3% aqueous solution, 10% solution of potassium hydroxide (KOH), 10% solution of FeSO4, and vanillin) independently and directly on the tissue of freshly collected mushrooms and observing the color changes immediately and after 4–5 min (Khaund & Joshi, 2014; Petrini et al., 2009).
The mushroom basidioma anatomy was observed under a compound microscope (Model No. Olympus CX31) after sectioning and staining with lactophenol cotton blue. Images were captured using a camera attached to a microscope.
For scanning electron microscopy (SEM), the samples were transferred to a Petri dish and immersed in 10 mL of water to rehydrate the tissues around the spores. Thereafter, the spore samples were incubated in the following ethanol series with 2% glutaraldehyde for 15 min each: 30, 50, 70, 80, 90, 95, and 100%. The samples were centrifuged, and the supernatant was collected. The sample was then placed in a Critical Point Dryer (CPD) and mounted. Finally, spores were prepared for SEM observation. The samples were coated with gold using a sputter coater. A focused image of the sample was used as the starting point under a scanning electron microscope. The magnification of the image was increased to a level close to the maximum, and the image was re-focused. A high-resolution SEM image was captured using an attached camera. All data or characters from macroscopic, microscopic, and biochemical reactions were compared with standard keys of mushrooms (Jordon, 1999; Pacioni, 1981; Phosri et al., 2007; Purkayastha & Chandra, 1985), and phenotypic mushroom identification was conducted.
For molecular identification of mushroom species, DNA was isolated from fresh basidioma using the modified hexadecyltrimethylammonium bromide (CTAB) method (Doyle & Doyle, 1987). In brief, 100 mg sample was frozen in liquid nitrogen and ground using 500 µL lysis buffer [CTAB 3% (pH 7.5), 100 mM Tris HCl, 20 mM EDTA (pH 7.5) 1.4 M NaCl2, 0.2% polyvinyl pyrolodine] and micro pestle in Eppendorf tubes. The sample was then incubated at 64–65 °C for 1 h in a water bath and vortexed at 10-min intervals. β-mercaptoethanol (20 µg/mL) was added to the tube, which was then incubated at 37 °C for 45 min. The sample was combined and centrifuged at 8000 rpm for 10 min at 4 °C using a cooling centrifuge. A 500 µL of chloroform: isoamyl Alcohol (24:1) was added to the solution, which was vortexed under chilled conditions. The solution was centrifuged at 12,000 rpm for 10 min at 4 °C in a cooling centrifuge. The supernatant was collected in fresh Eppendorf tubes. 500 µL of ice-chilled isopropyl alcohol (double volume) and 3 M Sodium acetate (1/10th volume) were added to the solution. Cold centrifugation was then performed at 12,000 rpm for 5 min at 4 °C. The supernatant was discarded, and the pellet was collected (containing DNA), dehydrated with 70% ethanol, and left to dry under laminar airflow. Thereafter, the pellet was resuspended in 50–100 µL of TE buffer and stored at 4 °C overnight. Isolated DNA was purified using a HiMedia Purification Kit. The purified DNA was electrophoresed via electrophoresis using 0.8% agarose gel at 100 volts for 2–3 h. DNA bands were observed using a UV transilluminator.
The ITS1–5.8S- ITS2 marker zone for the rRNA of isolated gDNA was amplified using a PCR (polymerase chain reaction) protocol modified from Gardes and Bruns (1993), with primers ITS-1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS4 (5′ TCCTCCGCT TATTGATATGC3′). In brief, PCR was conducted in a 50-µL reaction volume, which contained 1 ng/µL DNA template, approximately 1 µL; sterile distilled water, 38 µL; 10× Taq polymerase buffer with MgCl, 25 µL; 10 mM dNTP mixes, 3 µL; template DNA, 1 µL; ITS1 forward primer, 1 µL; and Taq DNA polymerase, 1 µL. The following PCR cycling program was employed: initial denaturation at 96 °C for 2 min followed by 35 cycles of denaturation at 96 °C for 1 min, annealing at 55 °C for 1 min, and extension at 72 °C for 2 min. The final extension at 72 °C for 10 min was terminated at the end of the amplification. The PCR product was sent to the SCI GENOME Laboratory, Kerala, for sequencing. The obtained sequence was compared with other related sequences using a BLAST search in GenBank (NCBI) (Ghosh et al., 2022; Podder & Ghosh, 2019) and submitted to GenBank, NCBI (Baltimore, USA).
Biodiversity Analysis and Mushroom Extraction in Different Solvents
Biodiversity Analysis
Fifteen study zones were selected from three forests in the Bankura district. Thus, 60 quadrats were used in this study. For mushroom biodiversity, quadrats (50 m × 50 m) were randomly selected, and the mushroom diversity was determined. Mushroom diversity was analyzed using the Shannon–Weaver Biodiversity Index (H) (Shannon & Weaver, 1962): H = −∑ (ni/N) loge (ni/N), where ni is the individual number of species i, and N is the sum of ni.
Mushroom Extraction in Different Solvents
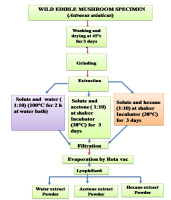
Mushroom extraction was carried out using the most polar solvent (hot water), semipolar solvent (acetone), and nonpolar solvent (hexane). The collected basidiomata were washed, dried, and crushed into powder using a grinder machine. Thereafter, 10 g of powder was poured separately into hot water, acetone, and hexane in a 1:10 ratio. For the hot water extract (HWE), a mixture of mushroom powder and water was boiled at 100 °C for 2 h in a water bath (Chen et al., 2016). However, for the acetone and hexane extracts, the mixture of mushroom powder and the solvent was shaken on a shaker at 150 rpm for 1 h, and then it was placed in an incubator set to 38 °C for 72 h. Each extract was filtered through Whatman No. 4 filter paper and Whatman No.1 filter paper. The filtrates were centrifuged at 3000 rpm for 15 min, concentrated through evaporation using a rotary evaporator at 50 °C, and lyophilized into powder using a lyophilizer (Figure 1). The extracts were stored at −20 °C for further use.
Qualitative Tests of Different Solvent Extracts of Mushroom Fruit Body for Mycochemicals
Flavonoid
Briefly, 0.2 g of extract was dissolved in 4 mL of distilled water in a test tube. Thereafter, diluted sodium hydroxide (1 M) was added to the mixture, resulting in a yellow solution that turned colorless following the addition of a few drops of dilute acid (10% hydrochloric acid), confirming the presence of flavonoids (Hossain et al., 2013).
Terpenoids
Approximately 0.2 g of extract was dissolved in a mixture of 2 mL of chloroform and 3 mL of concentrated sulfuric acid. A reddish-brown color eventually appeared run, indicating the presence of terpenoids (Mujeeb et al., 2014).
Cardiac Glycosides
Briefly, 0.2 g of the extract was combined with 2 mL of glacial acetic acid and one drop of FeCl3. This mixture was then added to 1 mL of concentrated H2SO4. When the upper layer and the interface between the two layers turned bluish-green and reddish-brown, respectively, this indicated the presence of cardiac glycosides (Auwal et al., 2014).
Steroids
The crude extract (0.2 g) was treated with a few drops of acetic acid anhydride in a test tube. Concentrated H2SO4 was carefully added dropwise using the inner wall of the test tube. The presence of a brown ring on the surrounding surface indicated the presence of steroids (Gul et al., 2017).
Saponins
The extract (0.2 g) was mixed with 5 mL of distilled water in a test tube. The solution was shaken vigorously for approximately 5 min and then incubated for 30 min. The formation of a honeycomb froth indicated the presence of saponin (Auwal et al., 2014).
Alkaloid
Briefly, 0.2 g crude extract was weighed in a separate test tube and warmed in 2% sulfuric acid for 2 min. In a separate test tube, the solution was mixed with a few drops of Dragendroff reagent. The presence of an orange-red precipitate indicated the presence of an alkaloid (Auwal et al., 2014).
Carbohydrate
The extract (0.2 g) was dissolved in water (2 mL). Thereafter, 2–3 drops of Molisch’s reagent [3.75 g of α-naphthol in 25 mL ethanol (99%)] were added to the solution to form a layer without shaking the test tube. Thereafter, a drop of concentrated sulfuric acid (99%) was added to the mixture. The presence of a purple color, which indicates the presence of carbohydrates, was determined at the interface (Auwal et al., 2014).
Phlobatannins
Briefly, 0.2 g extract was dissolved in 2 mL of water and then added to 3 mL of dilute HCl (10%). A red precipitate indicated the presence of phlobatannins (Auwal et al., 2014).
Phenol
Briefly, 0.2 g of extract was dissolved in 2 mL of distilled water. The solution was then treated with two drops of 5% FeCl3. The production of a deep blue color indicated the presence of phenol (Talukdar et al., 2017).
Divergent Biochemical Assay for Antioxidant Content and Antioxidant Potentiality
Antioxidant Content
Total phenolics content assay
The total phenolic content (TPC) of various solvent extracts was measured using the Folin–Ciocalteu reagent method (McDonald et al., 2001). Briefly, 0.1 mL Folin-reagent Ciocalteu’s (0.5 N) reagent and 0.5 mL extract were mixed and incubated for 15 min at room temperature. A saturated sodium carbonate solution (2.5 mL) was then added to the mixture, which was then incubated at room temperature for 30 min. The absorbance was measured at 760 nm. The results are expressed as mg of quercetin equivalent (QEs) per g of mushroom dry weight and are presented as mean ± SD (standard deviation) of triplicate experiments.
Total flavonoid content (TFC) assay
The TFC of the extract was evaluated using a standard guideline suggested by Meda et al. (2005). Briefly, equal amounts of 2% AlCl3 (prepared in absolute methanol) and the extracts (1 mg/mL) were incubated in a glass tube at room temperature for 10 min. Thereafter, the absorbance was measured at 415 nm. The results are expressed as mg of quercetin equivalent (QEs) per g of mushroom dry weight and are presented as mean ± SD of triplicate experiments.
Total ascorbic acid content assay
The total ascorbic acid content (TAAC) was determined using the Folin–Ciocalteu reagent method proposed by Jagota and Dani (1982). Briefly, 0.5 mL extract solution (1 g extract in 1 mL ethyl acetate) was quickly mixed with 0.8 mL of 10% trichloroacetic acid and refrigerated for 5 min before centrifugation at 3000 rpm for 5 min. Thereafter, 0.2 mL was retrieved from the mixture and mixed with 1.8 mL of distilled water to a final volume of 2 mL. Finally, 0.2 mL of diluted Folin–Ciocalteu (1:9) was added to the diluted extract mixture. After a 10 min incubation at room temperature (27 °C), the absorbance at 760 nm was measured. Finally, the results are expressed as milligrams of ascorbic acid equivalents (AAEs) per gram of mushroom extract, with the mean standard deviation (SD) of three replicates.
Antioxidant Activity Assay
DPPH Assay
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity (RSA) was determined using a modified method described by Bondet et al. (1997). Ten different concentrations (10–100 µg/mL) were prepared from the mushroom extract, and 900 µL DPPH (0.1 M) was added to each. The DPPH solution was prepared by adding 2 mg DPPH powder to 50 mL ethanol (0.1 M). After 2 h of incubation in the dark, the sample was subjected to a spectrophotometric study at a wavelength of 517 nm, and the absorbance was recorded. The percentage of DPPH reduction was calculated using the following formula:
Where Ac is the absorbance of the solvent (control) and As is the absorbance of the sample.
Finally, the IC50 (50% of inhibition) value was calculated.
. Results
Habitat Study and Soil Analysis
The collected mushrooms were rhizospheric and ectomycorrhizal under the roots of Shorea robusta, Modhuca indica, Petrocarpus marsupium, and Terminalia bellrica. When immature, the mushrooms were subterranean, but when fully matured, they were exposed by breaking the soil surface. The soil pH of the Beliatore, Gangal-ghati (G-ghati) forest was 6.3, and that of the Joypur forests was 6.5, 6.3, and 6.2, respectively. The soil analysis revealed that the organic carbon content of the collected soils ranged from 0.82 to 0.93%. The carbon content of the Beliatore soil samples was 0.93%, while that of the G-ghati soil sample was 0.87%, and that of the Joypur soil sample was 0.82%. The electrical conductivity (EC) of the three forest soil samples ranged from 81.58 to 86.72 µS. The value was 86.72 µS in the Beliatore forest, 84.29 µS in the G-ghati Forest, and 81.58 µS in the Joypur Forest. The available nitrogen content of the Beliatore forest sample was 220 kg/ha, while these of the G-ghati and Joypur forests samples were 140 kg/ha and 180 kg/ha, respectively. The available phosphorus content in the forest soils of the Bankura district ranged from 72.67 to 74.34 kg/ha, with values of 74.34 kg/ha, 73.54 kg/ha, and 72.67 kg/ha in the Beliatore, G-ghati, and Joypur forests, respectively. In the study area, available potassium ranged from 215.7 to 218.4 kg/ha, indicating a high potassium content in the three-forest soil sample of the Bankura district. The available potassium contents in the three forests (Beliatore, G-ghati and Joypur) were 215.7, 214.2, and 218.4 kg/ha, respectively, and the N:P:K ratios in these forests were 3:1:3, 2:1:3, and 2:1:3, respectively (Table 1).
Table 1
Mean values for the soil physico-chemical parameters and the N, P, and K ratios of the soil samples collected at selected quadrants of the Forests in Bankura district.
Identification of Mushroom
Phenotypical Identification
The fruiting season for this mushroom is May–August. The basidioma of this species was globose and smooth, with an unexpanded diameter of 10–16 × 8–14 mm and an expanded diameter of 14–23 × 8–12 mm. The gleba was initially white; however, when the spores matured, the gleba and its granulate outer peridium turned purplish chestnut. The exoperidium was slightly viscous and smooth, and the color was white, covering the endoperidium (Figure 2). The endoperidium is a thin membranous layer of tissue enclosing the spore mass in a 20–30 mm diameter sac, nearly round, with an irregularly shaped ostiole at the top, surface felt rough, whitish, and gray to brown color. Basidiospore mass was whitish when young and black and powdery at maturity. The spores were reddish-brown, roughly spherical with minute warts, measuring 7.5–11 µm in diameter. The mycelial layer consisted of branched hyphae that were 4–7 µm in diameter. The hyphae of the fibrous layer were 6–9 µm in diameter and branched, and the collenchyma-type layer contained branched hyphae 3–4 µm in diameter (Figure 3). Scanning electron micrographs provided a clear three-dimensional image and stereoscopic view of the mushroom spore and hyphal structures. Ornamentation on the spore surface and hyphal structure were clearly observed (Figure 4). The basidiospores showed extensive, dense, and rounded spine and were globose, ranging from 8.65–15.3 mm diameter. Other features included ornamentation, purplish chestnut color, very densely ornamented with rounded, narrow, tapered, occasionally coalescent spines 0.9–1.50 mm long.
Figure 2
Images of Astraeus asiaticus. (A) Young sporocarps collected from the field; (B) Young sporocarp; (C) Mature sporocarp.
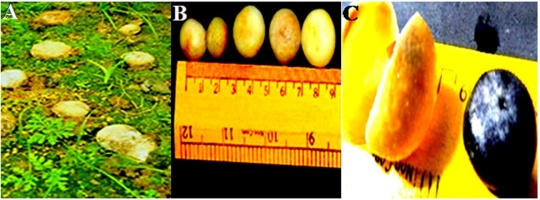
Figure 3
Images of the anatomy of Astraeus asiaticus. (A) Cross-section of the gleba; (B) Cross-section showing the arrangement of long thick-walled septate hyphal cells; (C) spore mass; (D) spore mass with basidiospore (40X).
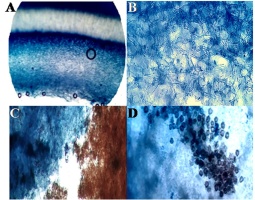
Figure 4
Scanning electron micrograph. (A) Scanning electron micrograph of the hyphae structure (bar = 1 µm); (B) Scanning electron micrograph of mature basidiospores displaying dense, rounded spine in groups (bar = 200 nm).

In the present biochemical identification study, no color change was observed when 10% KOH, 4% HNO3, Phenol, Vanillin, and 10% H2SO4 were employed (Table 2). The color change profiles generated for each species of wild edible mushrooms in the present study served as an additional tool to identify similar mushrooms.
Table 2
Color change profile based on the fruiting bodies of Astraeus asiaticus.
| Test | Colour changed observed |
|---|---|
| 10% KOH | None |
| 4% HNO3 | None |
| Phenol | None |
| Vanilin | None |
| 10% H2SO4 | None |
The collected mushroom was identified as Astraeus sp. based on the macroscopic and microscopic characteristics, biochemical reactions, and comparison with the standard keys of mushrooms (Jordon, 1999; Pacioni, 1981; Phosri et al., 2007; Purkayastha & Chandra, 1985).
Molecular Identification of Fungal Species
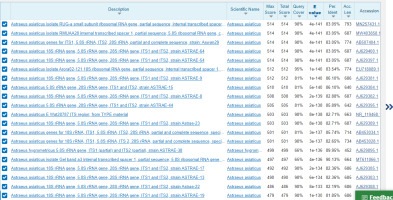
The mushroom sample was identified as Astraeus asiaticus using BLAST in the NCBI database based on query coverage (98.0%) and identity score (83.05%) (Figure 5) of sequence homology search. The nucleotide sequence of the ITS zone was submitted to Genbank, NCBI (Baltimore, USA) and published under Accession no MZ931288.1. The strain name is “Aa_skg.”
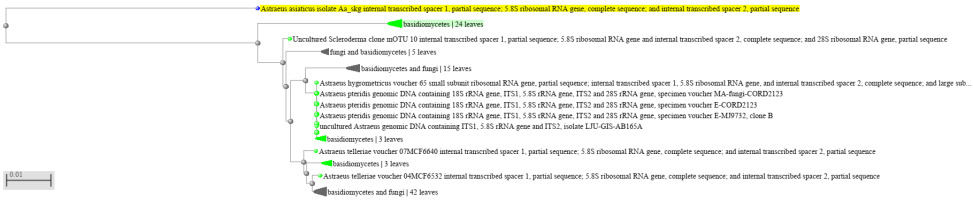
The phylogenetic tree (Figure 6) revealed that the test organism was closely related to Astraeus asiaticus. Thus, the wild mushroom was concluded to be a member of Astraeus asiaticus.
Biodiversity Analysis
The mushroom is terrestrial, grows under various trees, and is widely distributed in the three forests of Bankura district, West Bengal, India. Astraeus asiaticus can establish a symbiotic relationship with a wide range of trees. This ectomycorrhizal species forms a symbiotic relationship with trees, such as Shorea robusta, Modhuca indica, Petrocarpus marsupium, and Terminalia bellrica. In our study, the abundance of basidioma in this species was found to be associated with the roots of Shorea robusta; however, in the case of Madhuca indica, a moderate number of this species was found. Very poor or low numbers of this species were found in association with the roots of Terminalia bellrica and Petrocarpus marsupium. Further, no mushroom basidioma was found in association with Eucalyptus globulas and Acacia auriculiformis trees (Table 3).
Table 3
Association of Astraeus asiaticus with different trees.
| Trees | Fruit body association with trees |
|---|---|
| Shorea robusta | +++ |
| Petrocarpus marsupium | + |
| Terminalia bellrica | + |
| Acacia auriculiformis | − |
| Modhuca indica | ++ |
| Eucalyptus globulas | − |
The ecological diversity of Shorea robusta and Madhuca indica in the 60 quadrats of 15 zones of three forests was calculated using the Shannon diversity index formula.
The diversity index of Shorea robusta was 2.303 in the Beliatore forest, 2.178 in the Joypur forest, and 2.36 in the Gangajal–ghati forest (Table 4). Further, the diversity index of Madhuca indica was 2.25 in the Beliatore forest, 2.26 in the Joypur forest, and 2.29 in the Gangajal–ghati forest (Table 5).
Mushroom Extraction, Mycochemistry, Antioxidant Content, and Potentiality Study
Mushroom Extraction
The HWE had the highest extraction yield (0.47 ± 0.01%) for A. asiaticus (Table 6). The polarity index of water is 10.2. Accordingly, water dissolves many polar and ionic solutes, such as proteins, water-soluble carbohydrates, and mineral salts. In contrast, the nonpolar solvent, n-hexane, only dissolves nonpolar compounds, such as lipids, as its polarity index is 0.1, resulting in 0.32 ± 0.01b% crude product (HE). Acetone is a dipolar solvent; thus, it has an intermediate relative polarity. The polarity index of acetone is 5.1, resulting in a crude product, acetone extract (AE), of 0.42 ± 0.02%. The yields of HWE and AE were similar (P = 0.005); however, their yields differed from that of HE based on Duncan’s multiple test analysis (Table 6).
Table 6
Percentage yield of the crude products using different solvents.
Mycochemical Screening Using a Qualitative Test
Based on the mycochemical screening results (Table 7), the active ingredients in the HWE were flavonoids, cardiac glycosides, saponins, alkaloids, carbohydrates, carbohydrates, and phenols. For acetone, the active ingredients were flavonoids, cardiac glycosides, saponins, alkaloids, and phenols; and for the hexane extract, the active ingredients were terpenoids, cardiac glycosides, saponins, alkaloids, and tannins. Phlobatannins and steroids were not detected in any sample. Major active ingredient metabolites, such as flavonoids, alkaloids, phenols, and carbohydrates, were found in the polar HWE, while terpenoids were found in the nonpolar hexane extract.
Table 7
Mycochemical screening of the polar (water), dipolar (acetone), and nonpolar (hexane) solvent extracts of Astraeus asiaticus.
Antioxidant Content
Total phenolics, flavonoid, and ascorbic acid content
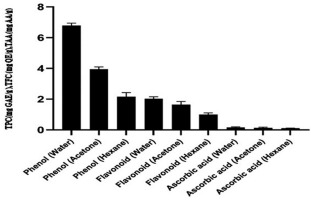
The total phenolics contents of the hot water, acetone, and hexane extracts were 6.8 ± 0.15, 3.95 ± 0.15, and 2.16 ± 0.26 mg GAE/g, respectively, and the TFCs were 2.03 ± 0.12, 1.65 ± 0.2, and 1.01 ± 0.08 mg QE/g, respectively. Figure 7 shows the ascorbic acid concentration in the mushroom extracts. The ascorbic acid content in hot water, acetone, and hexane extracts was low (0.167 ± 0.03, 0.14 ± 0.04, and 0.11 ± 0.005 mg AAE/g, respectively).
Figure 7
Total phenol, flavonoid, and ascorbic acid contents of Astraeus asiaticus in the hot water, acetone, and hexane extracts.
Antioxidant potential or scavenging activity based on the DPPH method
DPPH was used to evaluate the RSA of the mushroom decoction. DPPH is a stable free radical that exhibits idiosyncratic sorption at 517 nm. The absorption of these radicals decreases as antioxidants provide protons. As a measure of the magnitude of radical scavenging, a decrease in absorbance was confirmed. The free radical-scavenging capabilities of the extracts were calculated using the DPPH assay. A method by which antioxidants prevent lipid oxidation is free radical scavenging. The distillate method for scavenging DPPH free radicals can be used to assess the antioxidant activity of individual chemicals or extracts in a specific situation.
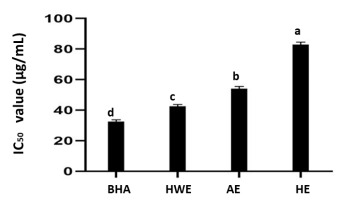
Figure 8 shows the DPPH RSA of all solvent extracts of this mushroom. The IC50 (50% of inhibition) values for the HWE, acetone extract, and nonpolar hexane extract were 42.54 ± 1.25 µg/mL, 54.06 ± 1.50 µg/mL, and 82.97 ± 1.58 µg/mL, while that of synthetic antioxidant BH was 32.41 ± 1.26 µg/mL.
. Discussion and Conclusions
Wild edible mushrooms, which serve as a good economic source for underprivileged residents of Bankura district, W.B., were collected to perform phenotypic and molecular identification studies and determine their diversity. This mushroom has been consumed as a good food in this area and adjoining districts. According to the literature, this genus of mushroom is distributed worldwide, especially in Africa, Asia, Australia, Europe, Mexico, North America, and South America (Pavithra et al., 2015). A literature survey revealed that Astraeus hygrometricus, A. asiaticus, A. morganii, A. sirindhorniae, A. odoratus, A. pteridis, A. smithii, A. telleriae, A. koreanus, and A. thailandicus have been discovered globally (Verma et al., 2019). In this study, both phenotypic and molecular identification of this mushroom were successfully performed. The relevance of nucleic acids as molecular markers applies to fungal taxonomy. PCR is an established habitual tool for field mycology. For example, internal transcribed spacer (ITS) regions are amplified by PCR and subsequently sequenced for molecular phylogenetic analysis of diverse mushroom species. The DNA sequences obtained via PCR are used for population genetics and biodiversity research on mushroom species (Ghosh et al., 2022; Izumitsu et al., 2012). In this study, both phenotypical and molecular (ITS1-5.8s-ITS-2 zone of rDNA) identification revealed that this mushroom belonged to the species Astraeus asiaticus, which was first identified in this district of West Bengal, India. Astraeus asiaticus is a new species of star-shaped gasteroid fungus that differs morphologically from other genera of star-shaped fungi. This genus does not possess a distinct peristome or columella. Further, the spores of this genus are larger than those of Geastrum, and its capillitial hyphae are longer and more branched (Phosri et al., 2014). Phosri et al. (2007) identified this species at the morphological and molecular levels. Our phenotypical and molecular results align with the findings of Phosri et al. (2007). Recently, Topno and Srivastava (2021) collected puff balls from Chota Nagpur Plateau (Jharkhand). These puffballs were phenotypically and molecularly (ITSs markers) similar to Astraeus asiaticus. Similarly, Vishal et al. (2021) collected two earth star mushrooms from the Sal forest of the Porahat forest division of Jharkhand state, India, and identified these mushrooms A. asiaticus and A. odoratus based on molecular studies (ITSs marker). From the GENBANK database (NCBI, Baltimore, USA), we identified five accession Nos for Astraeus sp. from 2008 to 2020: AJ629895.1 for A. hygrometricus from West Bengal, India in 2008; MN257431.1 and MT611066.1 for A. asiaticus from our neighboring state, Jharkhand, India in 2019 and 2020; and MN262679.1 and KJ847767.1 for Astraeus odoratus in 2019 and 2016.
SEM studies of spores and hyphae were performed according to the findings of Phosri et al. (2007). The study of biodiversity of this species of mushroom as ectomycorrhizan in this work in W.B. was the first work, but in other states of India, diversity work has been done to some extent in other species (A. hygrometricus) (Semwal et al., 2014; Topno & Srivastava, 2021; Verma et al., 2017; Vishal et al., 2021). After a distribution search of Astraeus species in India, this genus was found in the provinces of Himachal Pradesh, Uttar Pradesh, Uttarakhand, Madhya Pradesh, Chhattisgarh, Punjab, Karnataka, Kerala, Jharkhand, and Odisha, and in our state (Pavithra et al., 2015; Phosri et al., 2004; Pyasi et al., 2013; Semwal et al., 2014; Topno & Srivastava, 2021; Vishal et al., 2021). Some Asian varieties of A. hygrometricus are reported to differ from those found in North America and are recognized as new species, such as A. asiaticus and A. odoratus (Mortimer et al., 2012). In the present case, the Astraeus species was identified as A. asiaticus.
In our study, the plants supporting this mushroom were Shorea robusta, Madhuca indica, Petrocarpus marsupium, and Terminalia belerica. Such a finding is supported by other researchers (Harley et al., 1997; Verma et al., 2017). The ectomycorrhizal association of Astraeus with the roots of members of Pinaceae, Betulaceae, Fagaceae, Ericaceae, and Dipterocarpaceae has been reported (Wilson et al., 2012). The diversity index obtained in our study indicated that the ecological diversity of this mushroom was similar in the three forests in Bankura district, W.B. By analyzing the soil quality of these three forests, they were found to be more or less similar concerning NPK and pH status, aligning with the results of another study (Das, 2021). Mushrooms contain high amounts of antioxidant compounds (Chun et al., 2021; Shaffique et al., 2021; Wani et al., 2010). The antioxidant activity of A. hygrometricus was demonstrated previously (Mandal et al., 2015; Pavithra et al., 2016). Compounds, such as phenolic compounds, flavonoids, and ascorbic acids, have been found in other Astraeus species (Astraeus odoratus and Astraeus pteridis) (Arpha et al., 2012; Isaka et al., 2016; Lai et al., 2012; Mallick et al., 2016; Pimjuk et al., 2015; Rahi & Malik, 2016; Stanikunaite et al., 2008). In this study, the different parameters of the mycochemistry study of A. asiaticus were successfully analyzed based on the total phenolic, flavonoid, ascorbic acid, and antioxidant contents and the free RSA of this mushroom. In our experiment, all extracts of this mushroom had a high amount of phenolic compounds; however, the HWE had the best results. Similarly, this HWE had the highest antioxidant activity among the extracts. The antioxidant properties depend on phenolic compounds (Velioglu et al., 1998; Xu, 2013). The dietary intake of a substantial amount of phenolic compounds is important as they play the role of free radical terminators that reduce the incidence of many diseases, such as atherosclerosis, cancer, and heart diseases (Alothman et al., 2009; Xu, 2013). The antioxidant activity of many wild mushrooms is positively correlated with total phenolics (Lo & Cheung, 2005; Xu, 2013). Phenolic compounds have redox properties and antioxidant properties. As their free radical scavenging ability is facilitated by their hydroxyl groups, the total phenolic concentration can be used as a basis for rapid screening of antioxidant activity. Recently, Bouyahya et al. (2022) reviewed the role of phenolic compounds in improving the chemosensitivity of anticancer drugs. This study first time has compared the antioxidant activity of polar, semipolar, and nonpolar extracts of Astraeus asiaticus. So, mycochemical analyses should be performed to identify the active phenolic and flavonoid components. Ascorbic acid plays an important role as a protective antioxidant. In this study, the ascorbic acid contents in hot water, acetone, and hexane extracts were 0.167 ± 0.03, 0.14 ± 0.04, and 0.11 ± 0.005 AAEs/g, respectively, aligning with the previous results (Mau et al., 2002). The highest ascorbic acid content of 0.167 ± 0.03 AAEs/g, obtained using the HWE, might account for the better results found for their antioxidant activity. The HWE of A. asiaticus displayed potential scavenging activity (IC50 = 42.54 ± 1.25 µg/mL), comparable to that of the standard antioxidant, BHA. In general, the lower the IC50 value, the higher the antioxidant property. Accordingly, the HWE of Astraeus asiaticus was found to exhibit antioxidant attributes. These findings indicate that the HWE of mushroom species has a conspicuous effect on scavenging free radicals. Pavithra et al. (2016) reported that the antioxidant activity of A. hygrometricus is concentration-dependent and does not decrease after cooking. Herein, the HWE of A. asiaticus had the best antioxidant activity, which aligns with previous findings (Pavithra et al., 2016). Antioxidants are important compounds that help increase the resistance of the body to free radicals. Mycochemistry revealed that this mushroom was the source of some volatile compounds, such as 1-octanol, 1-octen-3-ol, and 1-octen-3-one, which had a “mushroom-like, earthy and pungent odor that was evident as an oily and moss-like smell upon opening the caps.” During the cooking of mushroom samples, furfural, benzaldehyde, cyclohexenone, and furanyl compounds are emitted (Mortimer et al., 2012; Verma et al., 2017). Recently, Isaka et al. (2016) isolated 17 known and seven new lanostane triterpenoids, such as astraeusins, 26-epi-artabotryol C1, and 26-epiastrasiaone, from A. asiaticus. To our knowledge, the current study is the first to assess the antioxidant content and activity of this edible mushroom species and its biodiversity in the forests of Bankura district.
Based on our findings, the species of Astraeus in the forest of the Bankura district was A. asiaticus. Further, this species was mycorrhizal associated with Shorea robusta and Madhuca indica. Based on the ecological diversity of this mushroom, A. asiaticus was associated with the above-mentioned specific plants. The mycochemistry of this mushroom revealed good quality and content of antioxidants, including phenolic compounds, flavonoids, and ascorbic acid, and good antioxidant activity (scavenging of free radicals). Therefore, this mushroom is a good source of valuable nutrients, including antioxidants, scientifically validating our eating habits. This work may fill the knowledge gap, to some extent, related to this wild edible mushroom (gift of nature), and encourages further assessments.