. Introduction
In recent years, the development of molecular methods has led to a significant increase in the number of taxa described as new to science (e.g., Guzow-Krzemińska et al., 2019; Kukwa et al., 2023; Nascimbene et al., 2023; Ossowska et al., 2022; van den Boom & Magain, 2020). At the same time, in many places around the world, including Poland, previously unrecorded species are still being discovered (e.g., Frisch et al., 2020; Khodosovtsev, 2023; Kossowska et al., 2022; Kukwa & Ossowska, 2023; Malíček et al., 2023; Matura et al., 2017; Ossowska et al., 2021; Ptach-Styn et al., 2024). Consequently, local lichen checklists must be constantly updated (Borgato et al., 2020; John et al., 2020; Tumur et al., 2021). According to the latest available data, the lichen biota of Poland comprises 1687 species (Fałtynowicz et al., 2024). However, there are still several lichen groups that need revisions, and that number will change.
The Megasporaceae is a lichen-forming fungal family, which includes eight genera: Aspicilia A. Massal., Circinaria Link, Lobothallia (Clauzade & Cl. Roux) Hafellner, Megaspora (Clauzade & Cl. Roux) Hafellner & V. Wirth, Sagedia Ach. (Nordin et al., 2010), Teuvoa Sohrabi & S. Leavitt (Sohrabi et al., 2013), Aspiciliella M. Choisy (Zakeri et al., 2017) and Oxneriaria S.Y. Kondr. & Lőkös (Haji Moniri et al., 2017). Most of the family representatives are saxicolous or terricolous species and are often widely distributed. Although taxonomic revisions continue resulting in the recognition of new species (Fryday et al., 2021; Iqbal et al., 2023; Lee et al., 2022; Sohrabi et al., 2023), Megasporaceae is still perceived as taxonomically complicated, with species difficult in the identification due to the inconspicuous, crustose thalli with limited morphological and chemical characters (Zakeri et al., 2019). The most important features of the family are crater-like, urceolate or lecanorine apothecia, asci with a non-amyloid tholus, the ‘Caesiocinerea-green’ pigment (Meyer & Printzen, 2000) present in the epihymenium, simple and hyaline ascospores, and branched, anastomosing and swollen at tips (moniliform or submoniliform) paraphyses (Lumbsch et al., 1994; Nordin et al., 2010). Recognition of species within the family must be additionally based on the ascospore size, the number of ascospores in the ascus, length of conidia, secondary lichen metabolites, and habitat preferences (Szczepańska et al., 2023).
The occurrence and distribution of representatives of the Megasporaceae in Poland are still insufficiently known, as well as the presumed number of species present. In addition to these listed by Fałtynowicz & Kossowska in (2016), four new species (Aspicilia verrucigera Hue, Oxneriaria supertegens (Arnold) S.Y. Kondr. & Lőkös, Sagedia mastrucata (Wahlenb.) A. Nordin, Savić & Tibell and S. zonata Ach.) occurring in the country have been recently recorded (Szczepańska et al., 2023) and supplemented in the latest checklist of Fałtynowicz et al. (2024). Nevertheless, here we report two additional taxa of the family Megasporaceae new to Poland, which were discovered during the herbarium revision and the fieldwork.
. Material and methods
The study material is deposited in UGDA (University of Gdańsk), KRAM (Polish Academy of Sciences, Kraków), and WRSL (University of Wrocław) herbaria. Descriptions of the species are based on our own observations. The morphology and anatomy of the species were studied with dissecting and light microscopes, following routine techniques; for light microscopy, hand sections were made with a razor blade and mounted in water. In the case of apothecia, hymenium measurements were made in water, and ascospore measurements were made in 10% KOH. The presence of the epihymenium pigment “Caesiocinerea-green” was detected by color reaction after the application of 50% HNO3 (Meyer & Printzen, 2000). The TLC analyses were performed in solvents A and C using the standardized method of Culberson (1972) and following Orange et al. (2001).
Additionally, in both species, attempts were made to isolate DNA to a barcoding procedure and to confirm taxa identification. For Circinaria leprosescens, sequences of ITS rDNA were obtained; however, in the case of Aspicilia fluviatilis, due to the small amount of material and the age of the specimen, DNA isolation was unsuccessful. The genomic DNA was extracted using a CTAB method according to the standard protocol of isolation (Doyle & Doyle, 1987; Guzow-Krzemińska & Węgrzyn, 2000). The primers used for DNA amplification were ITS1F (Gardes & Bruns, 1993) and ITS4 (White et al., 1990). PCR reactions were carried out using the following program: initial denaturation at 94 °C for 3 min and 33 cycles of 94 °C for 30 s; annealing at 52 °C for 45 s; extension at 72 °C for 1 min and a final extension at 72 °C for 10 min. PCR was performed in a volume of 25 µl using StartWarm HS-PCR Mix (A&A Biotechnology, Poland). PCR products were purified using Clean-Up (A&A Biotechnology, Poland) according to the manufacturer’s instructions. The purified DNA was sequenced by the Macrogen sequencing service (http://www.macrogen.com). For both sequences, we have obtained two F and R strands, which we have aligned in Auto Assembler v. 1.4.0 (Parker, 1997). The newly obtained nucITS rDNA sequences of C. leprosescens were subjected to a BLAST search (Altschul et al., 1997) and then deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank). For haplotype network analysis, the only one C. leprosescens sequence available in GenBank was downloaded and aligned in Seaview (Galtier et al., 1996; Gouy et al., 2010), and the terminal ends were cut. The TCS network, including three nucITS rDNA sequences (Clement et al., 2002) was created using PopArt software (http://popart.otago.ac.nz) and modified in Inkscape (http://inkscape.org).
. The species
Aspicilia fluviatilis A. Nordin & Owe-Larss.
Lichenologist 43(1): 30. 2011.
Figure 1A–C.
Figure 1
Specimens treated. (A–C) Aspicilia fluviatilis (KRAM L-50841), scales 1 mm; (D–F) Circinaria leprosescens (UGDA L-62011), (D) – scale 2 mm, (E–F) – scales 1 mm.
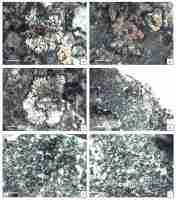
Morphology: Thallus lichenized, crustose, thin, radiating, rounded, light creamy yellow in the center to brownish yellow and grey on the edge, matt. Areoles flat to slightly convex, angular, smooth, 0.2–0.4 mm in diam. in center, elongated and branched in the outer part, 0.5–1 mm long. Prothallus invisible. Apothecia immersed, 1–2 per areole, 0.2–0.3 mm in diam., thalline margin conspicuous, thick, same color as the thallus or darker, brighter and radially incised on the inside edge, disc rounded to irregular, not pruinose or with white pruina. Hymenium colorless, 50–60 µm tall, with submoniliform paraphyses (3–5 globose apical cells), epihymenium olive-brown, N+ clearly green, K+ orange-brown (Caesiocinerea-green), hypothecium colorless. Asci 8-spored, ascospores hyaline, simple, narrowly ellipsoid, 15–20 × 5–10 µm. Pycnidia not seen in the examined material.
Chemistry: thallus K+ yellow turning red, P+ yellow, C–, KC–. Norstictic acid in the cortex, as well as in the apothecium discs pruina was detected, which is in accordance with literature data (Nordin et al., 2011). However, probably due to very low content, the red needles and color change were not observed in the cross-section of epihymenium.
Distribution and ecology:Aspicilia fluviatilis is a very rare species occurring mainly in subalpine and alpine habitats. It grows on siliceous rocks near running water or on the rocky scree under mountainsides. Single records of the taxon come only from Norway, Sweden and Russia (Nordin et al., 2011). What is interesting, during the revision of materials stored in KRAM and WRSL herbaria, two specimens collected in the Italian Alps in the 19th by Arnold were found. They were attributed to Aspicilia cinerea f. alpina Arnold. Perhaps the name is a synonym of Aspicilia fluviatilis, but this needs the revision of the type material. The single Polish specimen of this species was also found in KRAM herbarium under Aspicilia polychroma Anzi var. rubrireagens Asta & Roux. The species was collected on mylonite rock in the subnival belt in the Polish High Tatra Mountains.
Comments:Aspicilia fluviatilis belongs to the group of taxa with elongated, marginal areoles, occurring mainly in arctic and alpine zones (Nordin et al., 2011). Because of norstictic acid present in the cortex, it can be confused with A. granulosa A. Nordin and A. subradians (Nyl.) Hue, however these taxa have not been reported from Poland yet. Unlike A. fluviatilis, the main characteristic feature of A. granulosa is the granulose to subisidiate or verrucose central part of thallus (Nordin et al., 2011), whereas the typical feature of A. subradians is the very dark, almost black color of the upper surface and usually indistinct marginal areoles (Thomson, 1984). Additionally, A. fluviatilis can be distinguished from both species by pruinose discs with pruina containing norstictic acid and thalline margin with a white inside edge.
The specimen described herein was found in a single locality in the High Tatra Mountains, determined as A. polychroma var. rubrireagens, and reported as a taxon new to Poland (Flakus, 2007). Despite similarities in secondary chemistry, the two species are different in some characters. Aspicilia polychroma, including var. rubrireagens always has areolate or verrucose-areolate, not radiating thallus without any visible, elongate marginal areoles, and not pruinose, black disc. What is more, it usually occurs on slightly calciferous rocks in neutrophilic habitats (Szczepańska et al., 2023).
Specimen examined: Poland, West Carpathians, The High Tatra Mts, Szpiglasowa Przełęcz Pass, 49.198055°N, 20.042777°E, ATPOL grid square Ge-60, on mylonite rock, N aspect, slope 90°, mylonite area; the subnival belt, alt. 2107 m, 27 July 2003, leg. A. Flakus 971 (KRAM L-50841).
Additional material examined:
Italy, Alps, Trentino province, Lusia pass, Paneveggio-Pale di San Martino natural park, on porphyry rocks, 20 July 1888, leg. Arnold (KRAM L-996; WRSL-7949).
Circinaria leprosescens (Sandst.) A. Nordin, Savić & Tibell
Mycologia 102 (6): 1346. 2010 ≡ Aspicilia leprosescens (Sandst.) Hue, Nouv. Arch. Mus. Hist. Nat. Paris 5 sér. 2(1): 113. 1910.
Figure 1D–F.
Morphology: Thallus lichenized, crustose, 0.3–0.5 mm thick, areolate, smooth to irregularly cracked, light grey. Thallus surface granular with distinct, rounded, convex, brownish-grey granules resembling soredia or short isidia. Prothallus usually absent. Photobiont chlorococcoid. Apothecia and pycnidia not seen in the analyzed material.
Chemistry: thallus K–, P–, C–, KC–, medulla I–. Aspicilin was detected in Polish samples, as reported by Smith et al. (2009) and Wirth et al. (2013).
Distribution and ecology:Circinaria leprosescens is a typical maritime and strongly nitrophilous species (Sheard, 1965), associated with siliceous seashore rocks, especially manured by birds in xeric (lightly sprayed by sea water) or supralittoral (rarely submerged in the lower regions but subject to heavy spray) zones (http://www.lichensmaritimes.org). The species is widely distributed and locally frequent. It has been reported mainly from Europe, including Denmark (Søchting & Alstrup, 2008), Finland, Norway and Sweden (Westberg et al., 2021), France (Roux, 2012), Germany (Wirth et al., 2013), Great Britain and Ireland (Smith et al., 2009), Italy (Nimis & Martellos, 2024), Netherlands (Aptroot et al., 1999) and Ukraine (Kondratyuk et al., 2021). In Poland, the species was found on stones at the shore of Pucka Bay.
Comments –Circinaria leprosescens had not been previously reported from Poland; however, it was mentioned as probably occurring in Western Pomerania (Nowak & Tobolewski, 1975). During the revision of Megasporaceae specimens stored in Polish herbaria, only one specimen of the species was found, but originating from Sweden. Due to very characteristic ecology and habitat requirements connected with salted water, this species is rather easily recognized. Nevertheless, it seems that the thallus appearance of C. leprosescens is not well defined. According to literature data, thallus surface is variously described as squamulate, papillate, granular, isidiate (Sheard, 1965; Smith et al., 2009), sorediate (Nowak & Tobolewski, 1975; Wirth et al., 2013) or forming globulose or flattened schizidia (Nimis & Martellos, 2024). Due to the morphological similarity (especially bright color and soredia-like granules), C. leprosescens may be confused with some species of the genus Lepraria Ach., although Lepraria thallus is completely ecorticate, leprose and different in the secondary chemistry (Smith et al., 2009). In some cases, C. leprosescens may resemble Lepra corallina (L.) Hafellner, because of the presence of granules, which possess pale grey thallus and short, cylindrical isidia on the thallus surface. Both taxa are, however, different in habitat preferences, apothecia and ascospores appearance, as well as secondary metabolites (thamnolic acid present in L. corallina) (Smith et al., 2009). Within the family Megasporaceae, C. leprosescens may be confused mainly with Aspicilia aquatica (Fr.) Körb. and Oxneriaria supertegens (Arnold) S.Y. Kondr. & Lőkös, occurring in humid habitats in the mountains and with Circinaria caesocinerea (Nyl. ex Malbr.) A. Nordin, Savić & Tibell, a common, saxicolous, lowland lichen (Smith et al., 2009; Wirth et al., 2013). However, in each case, careful analysis of morphological and anatomical features of the specimens, connected with habitat data, will lead to the correct identification of species.
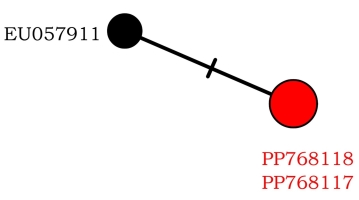
Two new nucITS rDNA sequences were obtained from our material. They show >98% similarity to the sequence of C. leprosescens from Sweden (EU057911), which was deposited by Nordin et al. (2010). On the haplotype network, species shows little variation, with only two haplotypes that differ by one nucleotide position (Figure 2).
Figure 2
Haplotype network showing relationships among ITS haplotypes of Circinaria leprosescens. The new sequences from Poland are in red.
Specimen examined: Poland, Pobrzeże Kaszubskie, E of Puck, 54.716182°N, 18.429469°E, stones rinsed with seawater, ATPOL grid square Ac-49, on stone, 19 March 2023, leg. M. Kukwa 24551 & 24552 (UGDA L-61207 & 61208); ibidem, 08 Aug. 2023, leg. M. Kukwa 25122 & 25124 (UGDA L- 63133 & 63135, GenBank PP768118); NE of Osłonino, by the cliff on the shore of Pucka Bay, 54.670731°N, 18.466133°E, ATPOL grid square Ac-59, on stones rinsed with seawater, 30 May 2023, leg. M. Kukwa 24810 (UGDA L-62011, Hb. K. Szczepańska 1431, GenBank PP768117).
Additional material examined: Sweden, Blekinge archipelago, Karlskrona city, 1875, leg. Svanlund (WRSL-5789).


