. Introduction
Specific crystalline deposit of various origins are often observed on the surface or in the interior of vegetative thalli and fruiting bodies of lichens. Qualitative and quantitative determination of such crystals can be very difficult without the use of specialized equipment or advanced chemical methods. There are three main types of crystals that can be found in lichens, namely crystallized products of secondary metabolism, metal oxalates (mostly calcium oxalates, CaOx) and calcium carbonate (CaCO3) derived from the rocky substrate on which the lichen grows. All of them are prominent in polarized light. However, the use of both standard microscopic techniques and common solubility tests does not always allow for a clear determination of the nature of crystals and their classification into a certain chemical group.
The synthesis of various unique secondary metabolites is a physiological peculiarity of lichens resulting from symbiotic relationships between mycobiont and photobiont. They are produced by mycobiont from primary metabolites but are not directly involved in the basic metabolism (Huneck, 1999). These substances can be deposited in the lichen thalli as extracellular fine crystals on the outer surfaces of the hyphae (Molnár & Farkas, 2010). Active lichen secondary metabolites could protect against herbivores, pathogens, competitors, and external abiotic factors, such as high insolation or heavy metals pollution (Huneck, 1999; Molnár & Farkas, 2010; Osyczka et al., 2021). The identification of these substances has been widely used in the taxonomy of lichens and various, more or less advanced methods, are involved in their determination (Huneck & Yoshimura, 1996; Orange et al., 2001). For example, the color and form of the crystallized substances, as well as their solubility in K and N chemical reagents (10% solution of KOH in water and 35% solution of HNO3, respectively), are essential taxonomic features in the genera Lecanora s.l. and Micarea (Launis et al., 2019; Śliwa, 2007).
Distinguishing between various kinds of Ca-containing crystals is rife with difficulties. As with all living organisms, calcium oxalates can be found in lichens in the form of variously crystallized aggregates. They are observed in external and/or internal parts of lichen thalli and fruiting bodies. Masses of such crystals are most often found on the thallus surface as the so-called pruina (e.g., Modenesi et al., 1998). Whereas the abundant deposit inside the thallus may even completely obscure the cortical or medullar layer in a cross-sectional view (Gaya, 2009). The crystals in the thallus interior are not readily examined since they are hard to reach and their initial diagnosis is very time-consuming. Specifically, CaOx crystals are stocks of calcium salt of oxalic acid produced by the lichenized fungus and are not the result of the secondary metabolism of lichens (Pinna, 1993). One of the functions of oxalic acid is to dissolve the rock substrate on which lichen grows. It has long been observed that the amount of CaOx in the thallus may be positively correlated with the amount of Ca in the host substrate (Syers et al., 1967). The role of oxalate crystals is to protect lichens against excessive insolation (Modenesi et al., 1998) and contribute to ensuring proper water balance (Wadsten & Moberg, 1985). These crystals can also help lichens neutralize the negative effects of various kinds of pollutants. For example, it was reported that the amount of pruina covering the thallus surface may be related to the degree of SO2 air pollution (Modenesi, 1993). Evidence was also provided to suppose that calcium oxalate crystals could contribute to heavy metal detoxification (Osyczka et al., 2018). Wadsten and Moberg (1985) reported two mineral forms of calcium oxalate hydrates, i.e., weddellite (CaC2O4 ⋅ (2+x)H2O; COD) and whewellite (CaC2O4 ⋅ H2O; COM). The former are associated with dry areas and can serve as a water source for lichens, while the latter are associated with moist habitats. According to Ručová et al. (2022), the occurrence of the two types of crystals is also related to the morphology of lichen thalli, with weddellite found in Cladonia furcata podetia exposed to the open environment and whewellite on C. foliacea squamules which form moisture-preserving microenvironments.
The CaOx crystals in lichens are studied mainly using X-ray analysis (detection of COD and COM crystals type) or scanning electron microscope techniques. By using more straightforward methods, a 10% aqueous solution of sulfuric acid can be applied to roughly determine CaOx crystals. This acid dissolves the crystals, and then the needle-shaped calcium sulfate precipitates (Timdal et al., 2017). Oxalate crystals were also treated with potassium hydroxide (KOH) and concentrated nitric acid (HNO3) (e.g., Wilk, 2012). However such tests turned out to be non-specific because CaCO3 crystals also demonstrated the same solubility in these reagents. Basically, CaOx crystals can be easily recognized using Yasue’s solubility test (Yasue, 1969); acetic acid dissolves CaCO3 but has no such effect on calcium oxalate crystals. However, this method can also be problematic since the test may give a positive result with other metal salts (Krajanová, 2023; Orange et al., 2001). Yasue’s solubility test has found practical application in lichen taxonomy (e.g., Giordani et al., 2003), but in spite of this, the test is used quite sparingly.
Through this article, we intended to present the problem related to the presence of various crystal deposits in lichens in view of taxonomic applications. The aim of our work was: (1) to attempt recognition of the crystals observed in the lichen thalli using simple methods commonly used in standard taxonomic procedures, (2) to draft a general classification of these crystals and to indicate the basic differences between them, and (3) to discuss the potential utility of features associated with crystals in taxonomic studies. This survey includes lichens from different genera, with particular attention given to those from Teloschistaceae. A crystalline outer coating or inner layer was, as a rule (or permanently) observed in representatives of selected taxa during routine taxonomic studies. This prompted us to undertake research aimed at resolving, at least partially, the problem in the context of traditional lichen taxonomy.
. Material and methods
Two sets of species were selected for the study. The first includes nine European species: Parmelia sulcata, Physcia dubia, P. dimidata, P. aipolia, Physconia distorta, Variospora flavescens and Verrucaria nigrescens, plus Calogaya pseudofulgensia and Pyrenodesmia albopruinosa. These lichens grow on limestone bedrock or tree bark. The crystals present in the above-mentioned species, with the exception of C. pseudofulgensia and P. albopruinosa, have been previously studied in detail using advanced chemical methods (Table 1 and references therein), and we therefore treated them as reference materials containing the identified crystal types. As part of our study, we carried out solubility tests on these CaOx crystals using basic chemical reagents traditionally used in taxonomic studies to know how they would react. In addition, we examined two interesting European calciphilous lichens, C. pseudofulgensia and P. albopruinosa, which also accumulate Ca-containing crystals (Gaya, 2009; Wilk, 2012) and have not been studied in detail before. The thallus of C. pseudofulgensia is filled with crystals, especially in the thalline medulla; so far, there has been no data on whether these are oxalate crystals or CaCO3 delivered from a calcareous substrate. The second species, P. albopruinosa, has characteristic crystals in the inner part of the apothecial cortex. Their presence was previously reported only by Wilk (2012); this study was an attempt to define them.
Table 1
Selected lichen species subjected to examination and distribution of crystals with their brief characteristics (PL – luminescent effect under polarized light).
| Lichen species (selected specimen) | Growth form | Substrate | Pruina | Crystal distribution | Crystal size and color | Solubility | Type of crystals | Relevant reference | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K | N | HCl | AA | ||||||||
| Calogaya pseudofulgensia (Wilk 2155; KRAM) | crustose | limestone | yes | thalline cortex | small, orange, PL+ (bright orange) | yes | no | no | no | crystalline secondary metabolites (anthraquinones) | |
| thalline cortex and medulla | large, hyaline, PL+ (white) | no | yes | yes | no | CaOx | |||||
| thalline medulla | large, hyaline, PL+ (white) | no | yes | yes | yes | CaCO3 (small amount) | |||||
| Parmelia sulcata (Nowak; KRAM-L-7751) | foliose | tree bark | no | inner part of thalline cortex | small, brown, PL+ (yellowish) | yes | no | no | no | crystalline secondary metabolites (atranorin) | Modenesi et al. (1998) Giordani et al. (2003) |
| Physcia aipolia (KRAM-L-61068) | foliose | tree bark | no | inner part of thalline cortex (upper part) | small, brown, PL+ (white) | yes | no | no | no | crystalline secondary metabolites (atranorin) | Giordani et al. (2003) Wadsten & Moberg (1985) |
| Physcia dimidata (Śliwa 1897; KRAM) | foliose | limestone | yes | thallus surface | large, hyaline, PL+ (white) | no | yes | yes | no | CaOx | Wadsten & Moberg (1985) |
| inner part of thalline cortex (upper part) | small, brown, PL+ (white) | yes | no | no | no | crystalline secondary metabolites (atranorin) | |||||
| Physcia dubia (Kiszka & Piórecki; KRAM-L-61109) | foliose | tree bark | no | inner part of thalline cortex | small, brown, PL+ (yellowish) | yes | no | no | no | crystalline secondary metabolites (atranorin) | Giordani et al. (2003) Wadsten & Moberg (1985) |
| Physconia distorta (Nowak; KRAM-L-L16476) | foliose | tree bark | yes | thallus surface | large, hyaline, PL+ (white) | no | yes | yes | no | CaOx | Giordani et al. (2003) Wadsten & Moberg (1985) |
| Pyrenodesmia albopruinosa (Śliwa 3026; KRAM) | crustose | limestone | yes | apothecial epihymenium and surface of proper margin | large, hyaline, PL+ (white) | no | yes | yes | no | CaOx | |
| inner part of apothecial cortex (thalline margin) | small, brown, PL+ (white) | no | yes | yes | no | cf. CaOx | |||||
| apothecial medulla | large, hyaline, PL+ (white) | no | yes | yes | yes | CaCO3 | |||||
| Variospora flavescens (Wilk 2129; KRAM) | crustose | limestone | no | upper part of thalline cortex | small, orange, PL+ (bright orange) | yes | no | no | no | crystalline secondary metabolites (anthraquinones) | Wadsten & Moberg (1985) |
| inner part of thalline cortex | large, hyaline, PL+ (white) | no | yes | yes | no | CaOx | |||||
| Verrucaria nigrescens (Nowak; KRAM-L-21371) | crustose | limestone | no | thalline medulla | large, hyaline, PL+ (white) | no | yes | yes | yes | CaCO3 | Syers et al. (1967) |
The second set includes 116 specimens of 30 species, mostly known from South America, that represent the poorly recognized group of Teloschistaceae, which we checked and verified for the presence of crystals using various reagents. These are saxicolous lichens growing mostly on siliceous rocks (100 specimens) or rarely calcicolous rocks (16 specimens) (see Table S1). They belong to the genus Wetmoreana and genera Aridoplaca, Calogaya, Caloplaca s.l., Cinnabaria, Gyalolechia, Squamulea, and Teuvoahtiana. The taxonomy, phenotypic characteristics and images of the studied members of Teloschistaceae are presented in Wilk and Lücking (2024).
Cross-sections of the thalli and apothecia were prepared by hand using a razor. Samples were observed under a Nikon SMZ 1270 dissecting microscope and a Nikon Eclipse 50i compound microscope. The presence of crystals was initially determined under polarized light. The following reagents were used to test the solubility of the crystals: potassium hydroxide (≅10% water solution of KOH; K), nitric acid (50% solution of HNO3; N), hydrochloric acid (2% solution of HCl) and acetic acid (5% solution of CH3COOH; AA). The reagents were applied by instilling under coverslips of microscopic slides with cross-sections of the examined thalli. In the case of the last two reagents, the solubility effect was verified immediately and thirty minutes after application, and the abundance of gas released during the solubilization process was assessed (following Yasue, 1969).
The compiled specification regarding the tested lichen specimens includes: (i) substrate on which the specimens occurred, (ii) presence and abundance of pruina on the surface of thallus and/or apothecia, (iii) internal location of crystal deposits in lichen thalli and/or apothecia (cross-sections), (iv) size and color of crystals under white light, (v) luminescent effect under polarized light, (vi) the susceptibility of crystals to be solubilized in the reagents used.
Since the chemical specificity of metal oxalates is not determinable with standard reagents, we used the term oxalate crystals (Ox) in a general sense; we assume that they represent primarily calcium oxalate crystals because this type of crystalline deposit is the most common in lichens.
. Results
Regarding the first set of species, our study focused only on the vegetative thallus. The exception was Pyrenodesmia albopruiniosa, which forms an endolithic thalli, and therefore, only apothecia were available for examination. In the studied taxa, crystals were located on the surface and in the inner part of the cortex and medulla in the thallus or apothecia. A large variability in the form of crystals was observed. Most often, the crystals were large in size, hyaline, and demonstrated white illumination under polarized light (Calogaya pseudofulgensia, Physcia dimidata, Physconia distorta, Pyrenodesmia albopruinosa, Variospora flavescens, Verrucaria nigrescens). Less frequently, the crystals were relatively small and brown and white or yellowish in polarized light (Parmelia sulcata, Physcia aipolia, Physcia dimidata, Physcia dubia, Pyrenodesmia albopruinosa). Additionally, small and orange crystals that appeared bright orange under polarized light were detected in Calogaya pseudofulgensia and Variospora flavescens (Figures 1–3, Table 1). Four types of crystals were revealed based on their solubility in chemical reagents (Table 2), namely, oxalate crystals (including calcium oxalate and cf. calcium oxalate crystals), calcium carbonate crystals, and crystallized products of secondary metabolism. The cf. calcium oxalate crystals demonstrated a similar solubility pattern to typical CaOx crystals and were found only in P. albopruinosa (Figure 3A–D). However, differences in size and color, as well as solubility in HCl, were noticed (crystals dissolved after 30 minutes of incubation).
Table 2
Type of crystals recognized in examined lichen species and their solubility in chemical reagents; for specification of reagents, see Material and methods.
Figure 1
Physcia dimidata – cross sections of the thallus in white (left row) and polarized (right row) light; (A, B) large hyaline crystals on the thallus surface (marked as 1 in Figure A) and small brownish crystals in the upper part of the thalline cortex (marked as 2 in Figure A) visible; (C, D) after HCl application only small brownish crystals visible; (E, F) after AA reagent application both crystals visible; (G, H) after K reagent application only large hyaline crystals visible; (I, J) after N reagent application only small brownish crystals visible. Scale bars: A, B = 20 µm; C, D, E, F, G, H, I, J = 50 µm.

Figure 2
Parmelia sulcata (A, B), Physcia dubia (C, D), Physconia distorta (E, F) and Verrucaria nigrescens (G, H) in white (left row) and polarized light (right row) – distribution of crystals in the cross-section is presented. Scale bars: A, B, E, F = 200 µm; C, D = 100 µm; G, H = 50 µm.
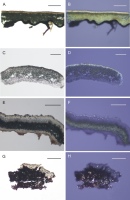
Figure 3
Pyrenodesmia albopruinosa (A–D), Flavoplaca flavescens (E, F), and Calogaya pseudofulgensia (G, H) in white (left row) and polarized light (right row) – distribution of crystals in the cross-section is presented. (B) Three types of crystals: 1 – Ox crystals, 2 – cf. oxalate crystals, and 3 – calcium carbonate crystals. (C, D) Small brownish cf. oxalate crystals located in the cortex of the apothecial thalline margin (marked by arrows; after first step HCl application). (E, F) Oxalate crystals located within the thallus cortex (marked by arrows). (G, H) Oxalate crystals located in the cortex and medulla of the thallus (marked by arrows in Figure H); a small amount of calcium carbonate crystals present in the medulla can be distinguished after treatment with chemical reagents. Scale bars: A, B, E, F = 50 µm; C, D = 20 µm; G, H = 100 µm.

In the case of the second set of species, a crystalline deposit not related to anthraquinones (commonly occurring in Teloschistaceae) was found in 71 of the 116 specimens examined. This deposit mostly referred to oxalate crystals as well as secondary metabolites of unknown origin (60 and 11 specimens, respectively). They were located mainly in the thalline medulla (66 specimens), thalline cortex (9), apothecial cortex (7), inner part of proper margin (=parathecium) (6), and apothecial algal layer (2) (Table S1). Medullary crystals were common in Wetmoreana species. In contrast, crystals in the thalline cortex were almost absent in Wetmoreana, except for two specimens of W. rubra (typically calcicolous species). Furthermore, in the case of W. rubra, crystals were also abundant in the apothecial cortex and parathecium. The presence of CaOx crystals in the thalline medulla and cortex was not regular in other species examined. Specimens of Cinnabaria boliviana, when associated with limestone rocks, were completely filled with CaOx crystals. Similarly, the studied taxa of Calogaya tended to accumulate these crystals in thallus cortex and/or medulla provided that the specimens grew on calcareous rocks. Moreover, the specific crystals visible under polarized light, soluble or not in K, but insoluble in N, AA, and HCl, were observed in specimens of W. bahiensis, W. brouardii, Gyalolechia gomerana. Since such a solubility pattern is not characteristic of Ca-containing crystals, the observed deposit probably represented secondary metabolites (further research is needed for unambiguous determination).
It was confirmed that the main differences between oxalate and calcium carbonate crystals are that the former are insoluble in AA. Moreover, in comparison to CaOx, calcium carbonate crystals quickly dissolve in HCl, releasing a large amount of gas, which can be quickly and easily detected by an observer. Moreover, we noticed that both CaOx and CaCO3 crystals are insoluble in K reagent while they are quickly soluble in N. Additionally, the mentioned solubility pattern does not apply to the secondary metabolites of the studied lichens because the latter are soluble or insoluble in K, insoluble in N and insoluble in HCl (Table 1, Table 2, Table S1).
. Discussion
The presence of crystals is increasingly being considered a potential diagnostic feature in various groups of lichens. Most often, they are the result of the secondary metabolism of lichens, and their solubility pattern does not correspond to that characteristic of CaOx and CaCO3 crystals, which are insoluble in K and soluble in N (Table 2). For example, members of the Lecanora subfusca group, in which the presence of crystals and their solubility serve as an essential diagnostic trait, produce crystals or granules in the epihymenium that are always soluble in K. On the contrary, the apothecial margin of those taxa often contains coarse crystals (insoluble in K and soluble in N), and according to Brodo (1984), they represent calcium oxalate crystals. Similarly, most of the members of the Lecanora dispersa group have apothecial cortex densely obscured by crystals prominent in polarized light that are insoluble in K and soluble in N; their abundance is related to the specific properties of the substrate in calcium-rich habitats (Śliwa, 2007). On the other hand, crystals found in epihymenium have diagnostic potential and have been divided into three groups according to their solubility characteristics: (i) K-insoluble and N-insoluble, (ii) K-soluble and N-insoluble, and (iii) K-insoluble and N-soluble (Śliwa, 2007). The presence of the first two types of crystals is linked to the occurrence of secondary metabolites in the species studied, while the latter type occurs in species in which no lichen substances were detected. Therefore, we assume that they represent oxalate crystals. Recently, the presence and distribution of crystalline granules within the apothecial and thallus structures have been proposed as a relevant species-level feature within the genus Micarea (Launis et al., 2019). All are K-soluble and probably represent the crystalline form of secondary metabolites rather than Ox crystals. K-soluble and N-insoluble crystals were also mentioned as diagnostic features for Myrionora (Palice et al., 2013) and likely relate to the accumulation of lobaric acid.
The occurrence of Ox crystals is a useful feature in taxonomic determination within the family Teloschistaceae. Lichens from this group usually produce crystalline secondary compounds (anthraquinones), which are K-soluble and N-insoluble. They are visible as orange crystals in the light under microscopy. However, there are taxa that additionally produce Ox crystals that clearly emerge under polarized light (Table S1). Oxalate crystals, which occur in the inner parts of thalli, in particular, have diagnostic potential in this group of lichens. As early as 1998, the medullary crystals insoluble in K and prominent under polarized light were defined as diagnostic features for the species Caloplaca appressa, C. eugyra, C. texana, and C. trachyphylla (Wetmore & Kärnefelt, 1998). Recently, two of these species, i.e., C. appressa and C. texana, and 28 other species, were subjected to additional detailed examination (Wilk & Lücking, 2024), which provided further evidence that medullary crystals are species-specific in this group of lichens. These crystals occur as a limited layer at the intersection between the algal and medulla layers, or they fill the entire medulla. The first type was observed in Wetmoreana ochraceofulva, W. variegata, W. appressa, and W. circumlobata, while the second in W. rubra and W. brachyloba, and, as a rule, in W. texana. There is a lot to suggest that the deposit refers to CaOx crystals; however, specimens from calcium-free substrates may contain other metal salts. In the case of W. texana, the presence of Ox crystals is not obligatory. They can often be found throughout the medulla of the thallus but are absent in the thalline cortex. We observed interesting variability in their abundance resulting from the type of habitat. The crystals are abundant in thalli exposed to strong sunlight (and then the thallus is intensively colored), while they are absent or sparse in thalli growing in shaded sites (and then the thallus is yellowish). Nevertheless, this observation requires confirmation by further studies.
The chemotypic trait that refers to Ox crystals present inside the lichen thallus may indeed be helpful in taxonomic diagnosis in the case of some pairs of very similar species; the best examples include Wetmoreana ochraceofulva vs. W. brouardii (Wilk, 2021) or Variospora flavescens vs. V. aurantia (Šoun & Vondrák, 2008). In contrast, there is no clear evidence to definitively conclude whether the externally located Ox crystals in the form of pruina are species-specific. According to Gaya (2009), the presence of pruina in representatives of Caloplaca s.l. may be genetically determined or related to specific environmental factors in which a given individual grows. The crystals in the hymenium of Caloplaca albopruinosa–alociza complex have aroused the interest of lichenologists (e.g., Clauzade & Roux, 1985; Muggia et al., 2008). According to our examination, apothecia of C. albopruinosa contain three types of crystals (Figure 3A–D). The surface of hymenium and apothecial margin contains Ox crystals (as it was showed by Muggia et al., 2008), the basal part of apothecium is filled by CaCO3 crystals, while within the cortex of thalline margin of apothecium, there are additional small brownish crystals (Wilk, 2012, and present study). The latter have a similar solubility pattern to Ox crystals, with the difference that they are soluble much longer in hydrochloric acid and have a slightly different color and size. Such a deposit probably refers to one of the morphological forms of Ox crystals (see Wadsten & Moberg, 1985). It can be assumed that the small brownish crystals within the apothecial cortex may serve as one of the useful diagnostic traits for C. albopruinosa. Within the group Teloschistaceae, Fulgensia s.l. appears as a complex of species that is particularly interesting due to the abundance of various crystals accumulating outside and inside the thallus (Gaya et al., 2008; Westberg & Kärnefelt, 1998); as yet, these crystals have not been studied in detail and require careful examination. Calogaya pseudofulgensia is morphologically similar to Fulgensia spp., and both tend to have a strong accumulation of crystals in their thalli. We noted that the thallus of C. pseudofulgensia contains large deposits of CaOx crystals, whereas only small amounts of CaCO3 crystals were observed. This is consistent with previous assumptions that typically calciphilic lichens have the ability to accumulate large amounts of calcium oxalates and consequently appear to be good indicators of calcium-rich substrates (Syers et al., 1967).
Regardless of taxonomic aspects, the adaptive role of a crystalline deposit in lichens is also intriguing. The function of the outer pruina is relatively easy to explain because such a crystal coating effectively reflects light and protects against excessive insolation (Gaya, 2009, see also Introduction). The role of crystals inside the lichen thallus in this respect is not so obvious. Perhaps one of the functions of crystals accumulated inside the thallus is protection against herbivores (Seaward et al., 1998). In the context of physiology, ecology, and taxonomy of lichens, there are many problems regarding the crystalline deposit that deserve to be studied in the future.
. Conclusions
Various crystals are commonly found in the thalli of many lichen species. Crystalline secondary metabolites have been relatively well studied. On the contrary, only a few studies have investigated oxalate crystals in terms of chemical properties, structure, and functional role in lichens. Through this article, we would like to draw attention to this important but neglected element of the lichen structure and highlight aspects that require further consideration. Oxalate crystals can be studied in the context of the ecological, physiological, and taxonomic properties of lichens. Their occurrence is often associated with specific environmental conditions; for example, it is known that various factors, such as sun exposure or air pollution, may influence the formation of crystals on the lichen surfaces. In parallel, in some taxonomic groups, Ox crystals can also serve as important diagnostic features. This applies, for example, to crystals located in the inner part of the lichen thalli in the form of a limited crystal layer (as in Variospora flavescens and Wetmoreana ochraceofulva). In contrast, the appearance of Ox crystal deposits as pruina on lichen thalli seems to depend on environmental conditions rather than the species. Crystals deposited directly on the surface or inside the lichen thallus may have different chemical structure; their chemical properties and function, as well as their influence on lichen physiology, require further investigation. The use of simple chemical reagents (such as K, N, HCl, and acetic acid) allows for basic crystal identification. Oxalate and CaCO3 crystals can be distinguished using an acetic acid reagent, which dissolves the latter. Furthermore, both types of crystals are soluble in HCl and N, but on the contrary they are not soluble in K. The studied crystalline secondary metabolites are characterized by insolubility in AA, HCl, and N reagents. However, their solubility in reagent K is not unambiguous and may vary depending on the species.
. Supplementary material
The following supplementary material is available for this article:
Table S1. Representatives of the genera Wetmoreana, Aridoplaca, Calogaya, Caloplaca s.l., Cinnabaria, Gyalolechia, Squamulea and Teuvoahtiana (Teloschistaceae) and distribution of the crystalline deposit not related to anthraquinones along with solubility characteristics in K, N, AA and HCl.


