. Introduction
Forest spring areas differ significantly from riparian forests, where the main factor shaping the vegetation is the hydrological regime of the stream and the characteristics of the entire catchment. Local factors such as insolation, exposure, substrate type, or altitude are more important for forest spring areas (Pielech, 2015). Springs are rich terrestrial and aquatic habitats that provide numerous ecosystem services disproportionate to the area they occupy. Their structure and character are crucial for the conservation and protection of mountain ecosystems (Beracko et al., 2022; Hidayat et al., 2023). An important factor influencing the biodiversity of spring areas is the local hydrological conditions. With the flowing water, minerals from deeper layers of the subsoil, which are inaccessible to plants, are brought to the surface. Spring areas may, therefore, be characterized by higher nutrients than adjacent areas (Bufková & Prach, 2006; Dahm et al., 1998). The chemical composition of water flowing from springs depends primarily on the type of bedrock (Chełmicki & Siwek, 2001). Therefore, even small mid-forest swamps fed by groundwater outflows may differ significantly from their surroundings. For that reason, forest springs usually also comprise local biodiversity hotspots.
Previous studies on the lichen biota and bryophyte flora in the Stołowe Mountains National Park (SMNP) have demonstrated that hydrogenic communities forming along rivers are refuges for valuable and rare species (Dimos-Zych, 2013; Wierzcholska et al., 2018, 2024). Despite the current knowledge that springs can be significant biodiversity hotspots within SMNP, the identification and assessment of such natural habitats have not been developed (Dyderski et al., 2018; Kącki et al., 2018; Pielech et al., 2018). Given that spring areas are relatively numerous in SMNP and are surrounded by diverse types of forest and non-forest vegetation, the study will focus on springs surrounded by spruce-alder forest communities with ash participation at varying degrees of alteration from their natural state.
Spring areas are unique environments characterized primarily by high atmospheric humidity. Poikilohydric organisms, such as lichens and bryophytes, benefit from these conditions, as the presence of dispersed water in the form of aerosols is crucial for all of their physiological processes, including rapid growth and both generative and vegetative reproduction (Glime, 2017b; Hawksworth & Grube, 2020; Spribille et al., 2022). These organisms are also recognized as playing a crucial role in creating biodiversity, especially in forest communities (Díaz et al., 2010; Kriebitzsch et al., 2013; Purvis, 2000). There are currently no lichenological studies on the lichen biota of springs in Poland, and only a few studies are available from Europe (e.g., Nascimbene et al., 2007). Research on lichens inhabiting freshwater environments has mainly focused on the species diversity of lichens associated with mountain rivers in the Western Carpathians and Bieszczady (Kiszka, 1998a, 1998b; Krzewicka & Galas, 2006; Krzewicka et al., 2017, 2020; Matura, 2020). These studies have demonstrated the significant role of rivers in shaping the biota of aquatic and hygrophilous lichens, which occur in the main river current, periodically flooded zones, and areas intensively sprayed with water. Many very rare and endangered species in Poland have been found in such habitats. Similarly, knowledge about the bryophyte flora associated with spring areas remains limited (Szmajda, 1979). Despite sparse knowledge regarding species diversity of lichens and bryophytes within spring areas, many species of protected and rare lichens and bryophytes are recorded here (Szmajda, 1979; Szweykowski, 1953). Springs are valuable areas not only because of their unique species composition and the proportion of protected and endangered species (Szmajda, 1979; Szweykowski, 1953; Wierzcholska & Plášek, 2006), but they are also a refuge for many species of invertebrates inhabiting bryophyte turfs (Glime, 2017a), whose development is closely dependent on the flowing water. These vulnerable habitats, which are often transition zones between wetter habitats like streams and adjacent woodland or grassland habitats, contain a specific combination of rare hygrophilous species (Cantonati et al., 2022). Because of climate change, land use practices that cause redirection of flows, and encroachment of alien species, springs are recognized as highly threatened ecosystems, and their state of exploration is described as insufficient in many aspects (Stevens et al., 2022). In view of the important role that springs play in forest habitats and in mitigating the effects of climate change, it is crucial to thoroughly study the biodiversity of these areas.
The study aimed to determine the species diversity of lichens and bryophytes in forested spring areas, assessing beta diversity (preferences for forest type) and alpha diversity (preferences to substrate type across all springs). We hypothesize that (1) the forest type will affect the richness of cryptogams, and (2) the greater species diversity of cryptogams in the springs is associated with a higher proportion of deciduous trees in the stand, particularly Fraxinus excelsior.
. Material and methods
The study area included forest springs within SMNP; SW Poland; 50.47°N, 16.35°E. These hydrogenic habitats are located at varying altitudes from 400 to over 700 m above sea level and are surrounded by montane and sub-montane coniferous forests, montane and sub-montane mixed forests, transitional fens (mires) and mountain hay meadows (acidophilus grasslands) (Figure 1). The vegetation within SMNP has been heavily transformed in the past by the replacement of the natural vegetation communities with coniferous monocultures. At present, spruce forests are still the continuous dominant vegetation type, undergoing a very dynamic process. Despite being significantly transformed from its potential natural vegetation, the study area still has a high conservation value. Springs within the SMNP form in two types of rocks: sandstones and at the boundary between sandstones and impermeable marls, where stratified springs arise. Stratified springs occur where the aquifer is intersected by the ground surface (Kowalski, 1980). Such springs appear at the boundary between the aquifer and the underlying impermeable layer (Hackett, 1998). Due to the significant hypsometric diversity of the Stołowe Mountains and the erosion of their slopes, often down to their base, erosion-slope springs predominate (Kowalski, 1980). Waters flowing from sandstone areas are acidic, while waters from marl areas are rich in calcium (Szmajda, 1979). Studies of riparian forest diversity in the Sudetes have shown that in forested spring areas, two types of riparian forests develop: ash spring riparian forest and alder-spruce spring riparian forest (Pielech, 2015). Both forest types have been recorded in the Stołowe Mountains.
Figure 1
The study area, including 22 plots surveyed in the Stołowe Mountains National Park (SW Poland; plot details see Table S1).
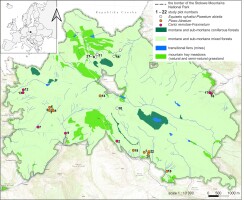
In all 22 established research plots (Figure 1), phytosociological surveys were conducted in August and September 2023. The spring areas were mapped in the field using a high-accuracy GPS receiver (Garmin 64s). The main criteria for determining the boundaries of the spring areas were vegetation characteristics and soil humidity. Classical phytosociological relevès were taken on each study plot using the old Braun-Blanquet seven-step scale (Braun-Blanquet, 1964). This made it possible to make a general characterization of the type of vegetation found in each spring studied. The phytosociological terminology was applied according to Chytrý (2013b), Douda (2013a, 2013b), and Mucina et al. (2016). Diagnostic species set for the identification of plant associations was used according to Chytrý (2013a) and Pielech (2015), and the taxonomic nomenclature followed by Danihelka et al. (2012). Phytosociological data of the study plots are available from the corresponding author upon request.
The study of cryptogams began on the plots determined in the field and phytosociologically identified. Research on the lichen biota and bryophyte flora was conducted from August to October 2023. During the field studies, for each study plot, all bryophyte and lichen species were listed concerning the substrate inhabited (soil – epigeic species; rotten wood like stumps, snags, and logs – epixylic species; bark of living trees – epiphytes; and stones – epilithes). For epiphytic species, data were collected specifying the name of the host tree (phorophyte) e.g., Abies alba, Acer pseudoplatanus, Alnus spp., Betula pendula, Corylus avellana, Fagus sylvatica, Fraxinus excelsior, Picea abies, Salix spp., and Sorbus aucuparia. The distinguished substrate types were analyzed in detail, and each substrate encountered within the study plot was examined.
We determined lichen species and bryophyte species in the field on macroscopic examination using hand lenses with light. If necessary, we collected samples for anatomical and chemical analyses in the laboratory. The collected lichenological and bryological material was morphologically and anatomically analyzed using standard microscope techniques (Nyholm, 1987, 1989, 1993, 1998; Smith et al., 2009). The presence of secondary metabolites in lichen thalli was determined by thin-layer chromatography (TLC) (Orange et al., 2001). The nomenclature of lichen species follows Fałtynowicz et al. (2024), and bryological nomenclature follows Hodgetts et al. (2020). Endangered (the threat categories) lichens and bryophytes in Poland were distinguished based on the work of Cieśliński et al. (2006) for lichens and Żarnowiec et al. (2004) for bryophytes. Protected species in Poland are classified according to the Regulation issued by the Minister of Environment (Dz.U. z 2014 r., poz. 1408; Dz.U. z 2014 r., poz. 1409). Relic species were identified following Cieśliński et al. (1996) for lichens and Stebel and Żarnowiec (2014) for bryophytes.
. Results
Based on the phytosociological surveys, the studied plots were classified into three plant associations. Two of them are riparian forests belonging to the Alnion incanae alliance – the ash-spring forest Carici remotae-Fraxinetum Koch 1926, and the alder-spruce spring forest Piceo-Alnetum Mráz 1959. The third is a spring forest from the Piceion abietis alliance, identified as the common horsetail and spruce association Equiseto sylvatici-Piceetum abietis Šmarda 1950 (Table S1). For each vegetation type, we calculated the total plot number and the total cover area. The largest number of study plots belonged to Piceo-Alnetum (n = 10), the next most abundant was Carici remotae-Fraxinetum (n = 7), and the least numerous was Equiseto sylvatici-Piceetum abietis (n = 5). According to the number of study plots, the total area of a given plant association had the same pattern: Piceo-Alnetum = in total 9,603 m2, Carici remotae-Fraxinetum = in total 7,370 m2, and Equiseto sylvatici-Piceetum abietis = in total 4,065 m2 (Table S1).
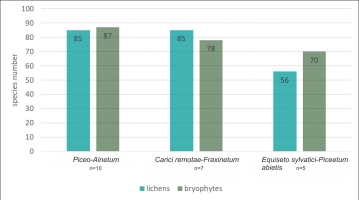
In total, across all types of spring forests (22 plots), we recorded 212 cryptogam species, including 110 lichens (Table S2), and 102 bryophytes – 78 mosses and 24 liverworts (Table S3). The community in which the most cryptogams were recorded was Piceo-Alnetum, with 173 species (86 lichens and 87 bryophytes, Figure 2). The next most abundant of this group of organisms was Carici remotae-Fraxinetum, with 164 taxa (85 lichens and 79 bryophytes). The lowest number of cryptogams was recorded in the Equiseto sylvatici-Piceetum abietis community – 127 species (55 lichens and 72 bryophytes).
Analyzing the species composition of the lichen biota found in the three studied spring types revealed that 38% of all species are present in all types of forest communities (Figure 3A). The remaining species were either common to two communities or exclusive to individual ones. The highest number of shared species was found between Piceo-Alnetum and Carici remotae-Fraxinetum communities, with 63 species. These two communities also had the highest number of exclusive species. Lower numbers of shared species were found between Piceo-Alnetum and Equiseto sylvatici-Piceetum (49 species), and Carici remotae-Fraxinetum and Equiseto sylvatici-Piceetum (45 species). These communities also had the fewest number of exclusive species (Figure 3A).
Figure 3
Venn diagrams presenting the number of species shared by plant communities, with lists of species occurring exclusively in particular habitat types; (A) number of lichen species, (B) number of bryophyte species. Lichen species exclusive to the plant communities – Piceo-Alnetum (14 species): Absconditella delutula, Agonimia flabelliformis, Chaenotheca brunneola, Ch. xyloxena, Cladonia cenotea, C. grayi, C. merochlorophaea, C. squamosa, Lecanora saligna, Porpidia crustulata, Trapelia glebulosa, T. placodioides, Trapeliopsis granulosa and T. pseudogranulosa; Carici remotae-Fraxinetum (18 species): Anisomeridium biforme, Arthonia ruana, Bacidia arceutina, Bacidina assulata, Chaenotheca trichialis, Fuscidea arboricola, Lecania croatica, Lecanora polytropa, L. thysanophora, Lecidella elaeochroma, Myriolecis persimilis, Parmelia barrenoae, P. submontana, Pertusaria pupillaris, Physcia stellaris, Polycauliona polycarpa, Scoliciosporum umbrinum and Toniniopsis separabilis; Equiseto sylvatici-Piceetum abietis (four species): Baeomyces placophyllus, Cladonia caespiticia, Lecanora chlarotera and Mycobilimbia tetramera. Bryophyte species exclusive to the plant communities – Piceo-Alnetum (nine species): Aulacomnium palustre, Ceratodon purpureus, Jochenia pallescens, Isothecium alopecuroides, Kindbergia praelonga, Leucobryum juniperoideum, Orthotrichum diaphanum, Plagiothecium cavifolium and P. undulatum; Carici remotae-Fraxinetum (nine species): Chiloscyphus pallescens, Pleuridium subulatum, Pohlia wahlenbergii, Pylaisia polyantha, Riccardia palmata, Scapania nemorea, Schistidium apocarpum, Sphagnum palustre, Ulota crispa; Equiseto sylvatici-Piceetum abietis (four species): Bazzania trilobata, Calypogeia neesiana, Plagiomnium elatum and Sphagnum squarrosum.
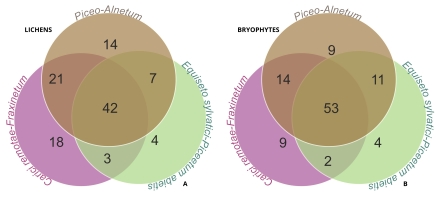
The analysis of the bryophyte species composition in the three examined spring types revealed that just over half of the species were common across all types. Specifically, 52% of all species are found in all types of forest communities (Figure 3B). Furthermore, species shared between pairs of communities also contained a high percentage of common bryophyte flora. The highest number of shared species was found between Piceo-Alnetum and Equiseto sylvatici-Piceetum communities – 64 species (63%). The other pairs, Piceo-Alnetum and Carici remotae-Fraxinetum, as well as Equiseto sylvatici-Piceetum, contained 67 and 55 common bryophyte species, respectively. The analyzed plant communities were also characterized by a low number of exclusive bryophyte species (Figure 3B).
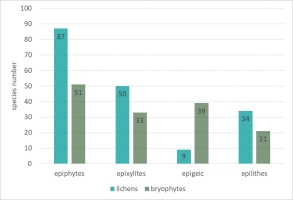
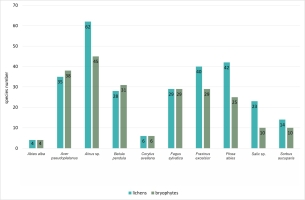
Epiphytes dominated the lichen biota observed within the spring areas, with 87 species recorded (Figure 4). The second largest group was epixylic lichens, with 50 species, which is related to the presence of large amounts of dead wood in various stages of decomposition. The epilithic lichen group comprised 34 species, as stones and rock outcrops occurred sporadically. Epigeic lichens had the fewest species, with only nine species recorded, due to the dense layer of mosses and herbaceous plants. Among the identified epiphytic lichens (growing on tree bark), the largest group was associated with Alnus sp. (62 species), followed by P. abies and F. excelsior (42 and 40 species each) (Figure 5). A high diversity of lichen species was also found on A. pseudoplatanus (35 species). The smallest number of species was noted on C. avellana (6 species) and A. alba (4 species), which is due to the singular occurrence of these trees in the field.
Like lichens, bryophytes most often colonized the bark of standing live trees – 51 epiphytic species were recorded (Figure 4). The other relevant substrate for the bryophyte flora was the soil. Because of the high moisture content of this substrate and the constant supply of surface water outflows, 39 species were recorded. Slightly fewer bryophytes were noted on decaying wood – 33 species. The smallest group was the epilithic bryophytes growing on stones and rock outcrops – 21 taxa were recorded. In the epiphytic ecological group (Figure 5), the bark of Alnus sp. (45 species), A. pseudoplatanus (38 species), B. pendula (31 species), F. sylvatica and F. excelsior (29 species each) were most readily colonized by bryophytes. The smallest number of species was noted on A. alba (four species).
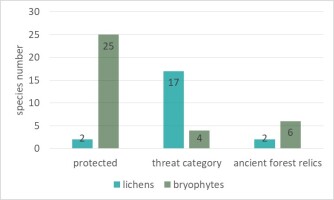
In the springs area, the presence of about 40 species of lichens and bryophytes under species protection and having different categories of endangered in Poland, were found (Figure 6, Table S1). In the lichen biota, only one species under strict species protection – Parmelia submontana, and one species under partial species protection – Hypogymnia tubulosa, were found. In total, 17 species endangered in Poland (Cieśliński et al., 2006) were recorded: critically endangered (CR) 1 species – Baeomyces placophyllus, extinct (EN) 6 species, e.g., Bacidia arceutina, Vulnerable (VU) 2 species, e.g. Anisomeridium biforme, Near Threatened (NT) 8 species, e.g. Pertusaria pupillaris and Trapeliopsis viridescens. Two species are relics of primeval forest (Cieśliński et al., 1996): Bacidia arceutina and Chaenotheca brunneola.
In the bryophyte flora, a total of 25 protected species were recorded (Figure 6), e.g., Homalia trichomanoides and Sphagnum fimbriatum, four species have threat categories: Vulnerable (V) 3 species, e.g. Ulota crispa, Rare (R) 1 species – Pulvigera lyellii. Six species are relics of primeval forests, e.g. Hypnum cupressiforme var. filiforme and Plagiomnium medium.
In the bryophyte flora, only one invasive species, Orthodontium lineare, was recorded. It spreads into forest communities at various stages of degradation, colonizing the bark of living trees and decaying wood. In the study area, it was recorded eight times across five research plots (see Table S3). It was found on the bark of living trees: B. pendula (3 records), A. glutinosa (1 record), and P. abies (4 records). In all plots where it occurred, the species was observed to produce sporophytes abundantly (Figure 7).
. Discussion
The three spring forest community types studied in SMNP showed considerable species diversity in both lichen and bryophyte. The very similar species number of these two groups of cryptogams confirms our previous conclusions that bryophyte and lichen species richness are positively correlated and that lichens and bryophytes can be used interchangeably in the field as habitat indicators (Wierzcholska et al., 2024). The three types of spring habitats studied were characterized by a high proportion of shared species, with 40%–50% of the identified lichens and bryophytes (Figure 2). This is likely due to the significant presence of common species associated with forest habitats. Of the springs examined, those classified as Piceo-Alnetum and Carici remotae-Fraxinetum appeared to be the richest in lichen and bryophyte species. These communities are composed of a large variety of tree species, such as P. abies, A. glutinosa, F. excelsior, A. pseudoplatanus, B. pendula, C. avellana, compared to the Equiseto sylvatici-Piceetum community, the poorest in cryptogams, which is mainly composed of P. abies. Among the recorded lichens and bryophytes are species very rare in Poland, in different threat categories (Cieśliński et al., 2006; Żarnowiec et al., 2004), e.g., Cladonia caespiticia, Parmelia submontana, and often stenotopic, such as primeval forest relics, e.g. Cheanotheca brunneola and Dicranodontium denudatum (Cieśliński et al., 1996; Stebel & Żarnowiec, 2014). The high number of shared species between Piceo-Alnetum and Carici remotae-Fraxinetum associations may be due to similarities in the classification and specific composition of the vascular flora of these forest springs, as well as their spatial structure. These two associations belong to the Alnion incanae alliance and their stands consist mainly of deciduous trees with varying proportions of spruce. In contrast, the distinctiveness formed here, through the low number of species common to the Equiseto sylvatici-Piceetum abietis compared to the previous associations, can be explained by being a spring forest from the Piceion abietis alliance, which is characterized by a negligible share of deciduous trees, acidic and nutrient-poor soil, and dominated by acidophilous species.
The results on the diversity of epiphytic lichen and bryophyte species clearly indicate that the species richness of epiphytes is related to the presence of deciduous trees (Figure 5). Despite the significant prevalence of P. abies in the study area (the total number of records for lichens and bryophytes is around 1,000) and the more scarce presence of Alnus spp., A. pseudoplatanus, and F. excelsior, it is the bark of deciduous trees that is most often overgrown by lichens and bryophytes. Similar findings from earlier studies showed that deciduous trees significantly enhance species richness and serve as important hotspots for epiphytic cryptogams in mountain temperate forests (Wierzcholska et al., 2018, 2024). The fact that mixed forest communities, composed of a variety of tree species, are richer in cryptogam species than communities dominated by conifers has also been found in works from other areas (e.g., Łubek et al., 2019, 2020; Moning et al., 2009; Ódor et al., 2013). An increase in biodiversity in forests with a greater diversity of tree species has also been observed for other living organisms (Gazda et al., 2015; Kinnoumè et al., 2024).
In the lichen biota and bryophyte flora, epiphytes are overwhelmingly dominant, and a slightly lower proportion of species on decaying wood were found (Figure 4). This pattern is related to the forested nature of the studied sites and the availability of heterogeneous substrates for cryptogams. Various tree species: A. alba, A. pseudoplatanus, Alnus spp., B. pendula, F. sylvatica, F. excelsior, and P. abies, have bark that differs in structure and water-holding capacity, creating a variety of microhabitats used by epiphytes (Barkman, 1958). Epiphytic species are the most abundant and diverse components of the lichen biota and bryophyte flora in the study area, which include a significant group of threatened and protected species. This indicates that the bark of living standing trees, especially deciduous ones, serves as a key habitat for maintaining the biodiversity of cryptogams in spring areas. Decaying wood in different stages of decomposition, in the shape of logs, stumps, dead-standing trunks, and root plate systems, increases the heterogeneity of substrates that are colonized by epixylic species. Among the lichens associated with dead wood in the study area were also two endangered lichen species: Cladonia caespiticia and Trapeliopsis viridescens. In spring areas, epigeic habitats are refuges of valuable, protected bryophyte species, especially those that form large mats, e.g., Sphagnum fallax or S. girgensohnii, and what is remarkable in this ecological group is the absence of terrestrial valuable lichens altogether. This was due to an overly dense moss and herbaceous layer. The only interesting lichen species that grew on the soil was Psilolechia clavulifera; it occurred, however, not directly on the ground but on the soil layer covering the upturned and exposed root plates of Norway spruce. This specific type of substrate is characteristic of naturally continuous forests, and it creates unique microhabitats for lichens (Cieśliński et al., 1996). Despite the limited presence of rocks and boulders in the studied plots, they are essential for certain epilithic lichens, which are rarely recorded and are variously threatened in Poland. Such species include Baeomyces placophyllus and Trapeliopsis viridescens.
The vulnerability to changes in the humidity of these habitats, despite the reduced impact of forest management, favors the intrusion of geographically alien species. This is likely due to the surrounding dominant spruce monocultures, where two out of the three invasive bryophytes in the Polish flora have been recorded – O. lineare (present in the spring communities and within the spruce monocultures) and Campylopus introflexus (spruce monocultures). Orthodontium lineare is a species native to the temperate zone of the Southern Hemisphere, was first reported in Poland in the 1980s and has since been steadily expanding its range (Żarnowiec et al., 2020). It is abundant in the Sudetes, particularly in spruce-dominated communities (Wierzcholska et al., 2018). It spreads through forest communities at various stages of degradation, colonizing the bark of living trees and decaying wood, and is in the process of expanding its range (Dyderski et al., 2022).
. Conclusions
Springs, due to their special microclimate related to high air humidity, are an important habitat for the preservation of the rich species diversity of lichens and bryophytes. This diversity is shaped not only by the humidity of the habitats themselves but also by the presence of heterogeneous substrates, mainly such as different species of deciduous phorophytes available for epiphytic cryptogams, wood in varying degrees of decay, inhabited by epixylic species, and rocks for lichens and soil for bryophytes. The obtained results indicate that within the ash and alder springs, there is an apparent increase in the species richness of lichens and bryophytes, which is related not only to the presence of F. excelsior but also trees such as Alnus spp., and A. pseudoplatanus. Springs in mountainous areas are important hotspots of cryptogam species diversity, especially species that are protected, endangered, and relics of primeval forests.