. Introduction
“Paprocie Serpentynitowe w Masywie Ślęży” ecological area which is found within the Ślęża Massif and the Oleszeńskie Hills, is a complex of 10 small areas, created for the protection of ferns of the Asplenium genus (spleenwort). Ślęża Massif, which belongs to the low mountains, is one of the seven mesoregions located within the Sudety Foreland and the highest area in this Foreland, rising above the surrounding plains to an altitude of approximately 500 m n.p.m. (Kondracki, 2011). The ecological studies is found in two municipalities – urban and rural commune Sobótka and the rural commune of Jordanów Śląski. The communes belong to the Wrocław County, which is within the administrative boundaries of Lower Silesian Voivodeship.
The Ślęża Massif is built from plutonic rocks, such as gabbro and granite, and metamorphic rocks, such as amphibolites and serpentinites (Podgórska, 2013). Serpentinite rocks, which are significantly found in the Oleszeńskie Hills, are formed by the transformation of ultramafic rocks, including peridotite and dunite. Antigorite is the main mineral in serpentinites. Serpentynites may additionally contain magnesite, apatite, or garnet, which are components of the Earth’s crust. Serpentinites are also characterized by a greenish or brown coloration with an admixture of black, which is often found as spots or stripes (Heflik, 2015). Serpentinite outcrops in the Massif are mainly dormant excavations of these rocks.
Serpentinites are unique and rare types of rocks that are only found in the Lower Silesia in Poland. Owing to their specific mineral composition and physicochemical properties, serpentinites are very special habitats for plants, bryophytes, and lichens. Serpentinite soils are deficient in plant nutrients, such as nitrogen, potassium, and phosphorus, but are characterized by a high concentration of heavy metals (iron, nickel, chromium, and cobalt) and low levels of calcium compared with the surrounding soils (Brady et al., 2005). These factors create a physiologically demanding environment for many organisms. Serpentinite soils are known to harbor very interesting and unique flora with high rates of endemism (Brooks, 1987; Whittaker, 1954). As a result, these soils have been the subject of many floristic studies in Poland (Szczęśniak, 2005), Europe (Brković et al., 2015; Tájek, 2010), and in other parts of the world (Kruckeberk, 1969; Morrey et al., 1989; Tyndall & Hull, 1999).
Most of the natural habitats, occurring in the Ślęża Massif, have been transformed into cultivated fields and urban areas, or have been covered by intensive forest management. Owing to these changes, almost the entire area of the Massif, formerly occupied by oak-hornbeam forests, is now overgrown by the coniferous-oak forest with the dominance of pine, as well as spruce at the higher sites. Only a small admixture consists of deciduous trees, such as oak, sycamore, beech, or alder (Fedyk et al., 2009). Natural xerothermic grasslands and characteristic for this region fern communities of the Asplenium genus are still present on a small areas in the Ślęża Massif. Taxa such as Asplenium adiantum-nigrum, A. cuneifolium, A. adulterinum, and A. onopteris var. silesiaca are found on the serpentinites (Public procurement No. WOF.261.3.2013, 2013). Habitats of Aspelnium were placed under protection as ecological areas in 2004 (Krajewski, 2012).
Owing to their unique character, serpentinite rocks have been an interesting object of lichenological studies (Hafellner, 1991; Paukov, 2009; Ritter-Studnicka & Klement, 1968; Sigal, 1989). The oldest data on lichens in Ślęża Massif date back to the second half of the nineteenth century. The German scholar, Bertold Stein, documented the presence of three lichen species on serpentinite rocks in the Lower Silesia (Stein, 1879). In 1896, another lichenologist, Eitner (Eitner, 1896) published his research, which revealed the presence of a new species in this area, Collema flaccidum (Ach.) Ach. In 1901, Eitner published another research from Lower Silesia, and revealed the presence of five lichen species. Both Stein’s (1879) and Eitner’s (1896, 1901) works covered a larger area than the Massif itself; thus, they provided information on few taxa in the area. In addition, these taxa were rare and endangered species. More detailed information of the lichen biota on serpentinites in Ślęża Massif was presented by Kossowska (2001), who demonstrated the occurrence of 68 species. An analysis of all the historical sources mentioned above, from 1879–2001, revealed the presence of 75 lichen species in the Ślęża Massif.
The aims of the current studies were to analyze the biodiversity of lichens inhabiting serpentinite rocks within the ecological area in the Ślęża Massif and to determine the abundance and health status of the individual taxa, which would describe the overall preservation status of the lichen biota in the study area, when combined with an analysis of the available historical data.
. Material and Methods
To study the lichen biota, three localities (Figure 1) from the complex of 10 that exist in Ślęża Massif ecological areas were selected. These localities are found within the boundaries of the Oleszeńskie Hills and represent diverse habitat conditions. The Oleszeńskie Hills are in the eastern part of the Massif. From the north, this area is surrounded by the villages Księgnice Małe and Sulistrowice, and to the south, it is surrounded by the Karolin and Oleszna. The boundary to the west is marked by Słupicka Pass, which separates the Hills from Radunia Mountain. From the east, the Hills are bordered by the Winna Mountain and the Przemiłów village.
Figure 1
Position of the examined localities of the “Paprocie Serpentynitowe w Masywie Ślęży” ecological area. Map source: https://mapy.geoportal.gov.pl/.
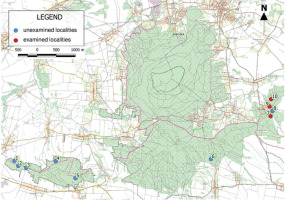
The first of the selected localities (No. 7; Figure 2A) is near the village Winna Góra, while the second (No. 9; Figure 2B) is in Przemiłów, and the third (No. 10; Figure 2C) is located north of Przemiłowo. The given localities numbers are the Asplenium ferns stands numbers which are officially approved within the established ecological areas. The total area of the examined localities was 1.51 ha. A detailed description of the localities covered in this study is provided in Table 1.
Figure 2
“Paprocie Serpentynitowe w Masywie Ślęży” ecological area. Locality No. 7 (A); locality No. 9 (B); locality No. 10 (C). Photo by M. Terlecka.

Table 1
Characteristics of the studied localities in the “Paprocie Serpentynitowe w Masywie Ślęży” ecological area.
Field studies were conducted in October 2019. At each of the three localities, fragments of lichen thalli were collected from randomly selected rock surfaces for later determination. Depending on the size and character of the locality, rocky surfaces were explored from 10 (locality No. 10) to 20 (locality No. 9). In addition, epixylic, epigeic, and epiphytic species growing within the boundaries of the ecological areas were studied. The material collected during the field study was identified in the laboratory. The morphology and anatomy of the specimens were studied with a dissecting and a light microscope, following routine techniques. Species that were difficult to determine by traditional methods were identified by secondary metabolite analysis using thin-layer chromatography (TLC). TLC analyses were performed in solvent system A using the standardized method of Culberson (1972) and following Orange et al. (2001).
Lichen was determined based on the following studies: Nowak and Tobolewski (1975), Wirth (1995), and Smith et al. (2009). Lichen species names are provided according to the Species Fungorum (http://www.speciesfungorum.org/). The categories of lichen endangerment are listed according to the “Polish red list of lichens” (Cieśliński et al., 2006).
Each of the taxa was assigned the following ordinal scales to capture the preservation status of the lichen biota:
A. The frequency class of the species was determined based on the number of localities and the total number of records at all localities, as follows:
Class I: Rare species that were noted in only one of the localities, with between one and six records (lichen thalli).
Class II: Frequent species that were noted at two localities, with records from seven to 14.
Class III: Common species that were noted in all three localities and had more than 14 records.
B. To determine the viability of each species, 10 randomly selected thalli were observed. For rare species with fewer than 10 records, all recorded thalli were analyzed. The viability of each species was subjected to summative assessment based on the morphology of the thalli as:
Very good (VG): Most of the specimens with a properly developed thallus and without visible damage.
Good (G): Most of the specimens with few minor thallus damages, such as necrosis and discoloration.
Bad (B): Most of the specimens with deformed thallus, which did not reach full size or had numerous damages.
C. To capture the dynamics of the lichen biota transformation, historical lichenologists’ materials on the presence of lichen in the serpentinic area in the Ślęża Massif were analyzed and compared with the obtained results.
Based on the analysis of these data, the individual taxa were classified in terms of their stability in occurrence on the habitat, as follows:
Stable (S): Species recorded by Stein (1879), Eitner (1896, 1901), Kossowska (2001), and Terlecka and Szczepańska (current study).
Probably in regression (R): Species recorded by Stein (1879), Eitner (1896, 1901) or Kossowska (2001).
Probably in progression (P): Species recorded by Kossowska (2001) and Terlecka and Szczepańska (current study).
The species first recorded in the serpentinic area in the Ślęża Massif through the current research was not clearly classified in any of the groups owing to insufficient data.
Based on information on the frequency class, the viability of the thallus, and the stability in occurrence on the habitat, the approximate preservation status of each species in the study area was determined as:
Very good: Common species (Class III), very good viability (VG), probably in progression (P).
Good: Frequent species (Class II), very good (VG) or good viability (G), probably in progression (P).
Moderate – rare species (Class I), very good (VG) or good viability (G), probably in progression (P) or stable (S).
Low: Rare species (Class I), bad viability (B), probably in progression (P) or stable (S).
Insufficient data: Species first recorded in the Ślęża Massif owing to the current studies, but cannot be classified due to the lack of data on its occurrence in the past.
. Results
Owing to the current studies carried out in the eastern part of the “Paprocie Serpentynitowe w Masywie Ślęży” ecological area, 47 lichen species were found. In locality No. 9 found in the village of Przemiłów, 32 species were recorded, while in locality No. 7, which is located on the slopes of the Winna Mountain, 17 lichen species were found. The smallest species diversity was found in Mount Gozdnik in locality No. 10, where 12 species were identified. Two of the recorded species, Psilolechia lucida (category LC – Least concerned) and Bacidina sulphurella (category NT – Near threatened), are on the red list of lichens in Poland (Cieśliński et al., 2006). No legally protected species, as defined in the Regulation of the Minister of Environment on the species protection of fungi (Regulation of the Minister of Environment, 2014), were found.
Most species found in the study area (77%) were classified as locally rare (Class I). Caloplaca subpallida, Cladonia ochrochlora, Placynthiella dasaea, and Rusavskia elegans were only found in one locality. The second class (Class II) consisted of frequent species (17%) that appeared in two localities. This class included Athalia holocarpa, Lepraria finkii, and Physcia tenella, among others. The last class (Class III), the smallest in terms of numbers, consists of common species, which were noted in all three localities, such as Candelariella vitellina, Physcia dubia, and Verrucaria muralis.
The viability of most species was found to be good. Minor damage to the thallus was observed in 33 species, including Acarospora fuscata, Myriolecis dispersa, and Lecanora muralis. A properly developed thallus and very good viability were found for 12 species, including Candelariella aurella, Physcia dubia, and Trapelia coarctata. The viability of the thallus of Phaeophyscia nigricans and Rusavskia elegans was determined to be bad. Further, their thalli were not fully developed or had visible damage.
Field and laboratory studies conducted in 2019 confirmed the presence of 29 species previously recorded in the Ślęża Massif by lichenologists. A total of 46 species were not found among previously recorded species. Moreover, none of the species recorded in the nineteenth century by Stein (1879) and Eitner (1896, 1901) were found. Nevertheless, 18 taxa that had not been previously noted in historical sources, were found, including a species recently discovered to be new in Poland, Caloplaca subpallida (Szczepańska et al., 2013). Based on an analysis of historical data, 46 species noted by Stein (1879), Eitner (1896, 1901), or Kossowska (2001) were classified as species “probably in regression” (R). The remaining 29 species, based on Kossowska’s (2001) and Terlecka and Szczepańska (current study) records, were classified as “probably in progression” (P). No species recorded by German researchers, Kossowska (2001) and Terlecka and Szczepańska (current study), which could be classified as “stable” (S), were found.
Based on the three factors discussed above (A. frequency class, B. viability, and C. stability in occurrence on the habitat), for each of the taxa, the preservation status in the eastern part of the Massif area was determined. In most cases, the lichen preservation status was described as “good” or “moderate.” “Good preservation status” was determined for nine species, while “moderate preservation status” were determined for 17 species. Only three species, the most common and numerous in terms of thalli numbers (namely Candelariella vitellina, Physcia dubia, and Verrucaria muralis), received a “very good preservation status.” None of the species was classified to have a “low preservation status.” For 18 species, the preservation status was noted as “insufficient data” due to a lack of records in earlier sources.
According to both historical and contemporary data, 93 lichen species were found in the Massif area. An inventory of the species recorded in the current study, together with their frequency class, viability, stability in occurrence on the habitat, and preservation status in the Massif, is presented in Table 2.
Table 2
Preservation status of the lichens noted during the studies conducted in 2019 in the “Paprocie serpentynitowe w Masywie Ślęży” ecological area.
. Discussion
Characteristic of Contemporary Lichen Biota and Historical Data
In the literature, the lichen biota connected with serpentinite rocks is usually not very rich (Hafellner, 1991; Gilbert, 2000; Purvis & Halls, 1996), which is explained by the high concentrations of Mg and Ni and the low availability of macronutrients (Bates, 1978). This paucity of lichens on serpentinites has been also observed in Poland (Bielczyk & Kossowska, 2015; Kossowska, 2001). The properties of serpentinite rocks promote the development of certain species of lichens, which are known to be ubiquitous and common on other types of rock substrates. The species characteristic of serpentinites include Acarospora fuscata, Amandinea punctata, Candelariella vitellin, and Lecanora muralis, which also dominate the lichen biota in the studied localities. Serpentinite rocks can often be overgrown by a mixture of siliceous, as well as calcicolous species (Ryan, 1988). In the study area, lichens that are more common on alkaline rocks include, Rusavskia elegans and Physcia caesia, among others, while those associated with siliceous rocks include Lecidea lapicida, Porina chlorotica, and Rhizocarpon ssp. However, many lichens found on serpentinites do not show a connection with just one type of substrate and may occur both on acidic and alkaline rocks (Bielczyk & Kossowska, 2015), such as Candelariella aurella, Flavoplaca flavocitrina, Myriolecis dispersa, and Lecanora muralis. In addition, in the study area, species known to be present on rocks rich in metals and minerals, such as Rhizocarpon distinctum and Trapelia glebulosa (Purvis & James, 1985), have also been found.
Despite the paucity of lichens mentioned above, data included in some papers prove that a higher number of lichen species exists on serpentinite rocks than on mafic rocks of adjacent areas (Paukov, 2009; Rajakaruna et al., 2012; Sirois et al., 1987). Such occurrence is explained by the slow colonization processes of serpentinite rocks by plants, which is convenient for inhabitation by saxicolous lichens (Favero-Longo et al., 2004). Moreover, the lichen biota growing on serpentinite rocks is sometimes considered to be a very specific, characteristic (Favero-Longo et al., 2004; Sirois et al., 1987) and favorable to the development of rare species (Gilbert & James, 1987; Kossowska, 2001; Sirois et al., 1987; Takala & Seaward, 1978).
Owing to the current studies conducted in the eastern part of the “Paprocie Serpentynitowe w Masywie Ślęży” ecological area in 2019, 47 lichen species were found. Compared to other studied places, where the number of species exceeded 100 (Gilbert & James 1987; Paukov, 2009; Sirois et al., 1987), the biota of the serpentine rocks of the Ślęża Massif seems to be rather poor. However, as the size of the examined area was only 1.51 ha, a relatively high species diversity of lichens can be considered to exist, compared to the number of all lichen taxa found in the area of the Ślęża Massif. Furthermore, the same species were rarely recorded in more than one of the examined locality, which indicates their unique and diverse lichen biota. Only three taxa were present in the three areas: Candelariella vitellina, Physcia dubia, and Verrucaria muralis. The lichen biota in the studied localities seem to be characteristic of serpentinite rocks and show comparable biodiversity to that of the other studied rocky areas in Lower Silesia (Kossowska, 2008; Kossowska & Szczepańska, 2020).
In the historical sources, from 1879–2001, the presence of 75 lichen species in the Ślęża Massif was highlighted (Table 3). In the current study, 29 species were confirmed. The share of species found again was approximately 30%. Studies by lichenologists in the past, covered a much broader study area. Stein (1879) and Eitner (1896, 1901) assessed lichen biota throughout the Lower Silesia. In the Ślęża Massif, the German researchers obtained their observations on the territory of the Kiełczyńskie Hills, located on the opposite side of the Massif, in relation to the ecological area described in this work. Kossowska (2001) described the presence of lichens in serpentinite rocks throughout Lower Silesia. In the Ślęża Massif itself, the author selected 14 locations that were observed; however, these areas differed from those currently being studied. Therefore, based on the significant differences in the size of the study areas and their different locations, as many as 46 species, reported in historical sources as occurring in the Massif area, were not found again during the current studies. In addition, taxa recorded more than 100 years ago by the Germans appear on the Polish red list of extinct and endangered species in Poland (Cieśliński et al., 2006). The species, Caloplaca rubelliana, is considered regionally extinct, while Collema flaccidum is considered a threatened species in the region. The third species (Caloplaca cf. atroflava), recorded in the Kiełczyńskie Hills by both German researchers, was found by Kossowska (2001) 100 years later. This species may not have been recorded in the current study owing to a difference in the location in the study area. Further, some of the previously recorded species for the Ślęża Massif could be extremely rare or very sensitive, which affected their ability to survive, despite only minor changes in their habitats.
Table 3
List of lichens recorded in the Ślęża Massif from historical sources.
| No. | Taxon | Stein (1879) | Eitner (1896) | Eitner (1901) | Kossowska (2001) |
|---|---|---|---|---|---|
| 1 | Acarospora fuscata (Nyl.) Arnold | - | - | - | + |
| 2 | Aspicilia cinerea (L.) Körb. | - | - | - | + |
| 3 | Aspicilia laevata (Ach.) Arnold | - | - | - | + |
| 4 | Athallia holocarpa (Hoffm.) Arup, Frödén & Søchting | - | - | - | + |
| 5 | Buellia aethalea (Ach.) Th. Fr. | - | - | - | + |
| 6 | Buellia ambigua (Ach.) Malme | - | - | - | + |
| 7 | Buellia stigmatea Koerber | - | - | - | + |
| 8 | Caloplaca atroflava (Turner) Mong. | + | - | + | + |
| 9 | Caloplaca rubelliana (Ach.) Lojka | + | - | - | - |
| 10 | Candelariella aurella (Hoffm.) Zahlbr. | - | - | - | + |
| 11 | Candelariella vitellina (Hoffm.) Müll. Arg. | - | - | - | + |
| 12 | Carbonea vitellinaria (Nyl.) Hertel | - | - | - | + |
| 13 | Catapyrenium squamulosum (Ach.) O. Breuss | - | - | - | + |
| 14 | Catillaria atomarioides (Müll. Arg.) Kilias | - | - | - | + |
| 15 | Circinaria caesiocinerea (Nyl. ex Malbr.) A. Nordin, Saviæ & Tibell | - | - | - | + |
| 16 | Cladonia chlorophaea (Flörke ex Sommerf.) Spreng. | - | - | - | + |
| 17 | Cladonia coniocraea (Flörke) Spreng. | - | - | - | + |
| 18 | Cladonia fimbriata (L.) Fr | - | - | - | + |
| 19 | Cladonia furcata (Huds.) Schrad. | - | - | - | + |
| 20 | Coenogonium pineti (Schrad.) Lücking & Lumbsch | - | - | - | + |
| 21 | Collema flaccidum (Ach.) Ach. | - | + | - | - |
| 22 | Flavoplaca flavocitrina (Nyl.) Arup, Frödén & Søchting | - | - | - | + |
| 23 | Hypogymnia physodes (L.) Nyl. | - | - | - | + |
| 24 | Lecanora bicincta Ramond | - | - | + | - |
| 25 | Lecanora campestris (Schaer.) Hue | - | - | - | + |
| 26 | Lecanora intricata (Ach.) Ach. | - | - | - | + |
| 27 | Lecanora muralis (Schreb.) Rabenh. | - | - | - | + |
| 28 | Lecanora polytropa (Hoffm.) Rabenh. | - | - | - | + |
| 29 | Lecidea fuscoatra (L.) Ach. | - | - | - | + |
| 30 | Lecidea tessellata Flörke | - | - | - | + |
| 31 | Lecidella anomaloides (A. Massal.) Hertel & Kilias | - | - | - | + |
| 32 | Lecidella asema (Nyl.) Knoph & Hertel | - | - | - | + |
| 33 | Lecidella carpathica (Fr.) Körb. | - | - | - | + |
| 34 | Lecidella stigmatea (Ach.) Hertel & Leuckert | - | - | - | + |
| 35 | Lepraria finkii (B. de Lesd.) R. C. Harris | - | - | - | + |
| 36 | Lepraria incana (L.) Ach. | - | - | - | + |
| 37 | Lepraria rigidula (B. de Lesd.) Tønsberg | - | - | - | + |
| 38 | Leproloma vouauxii (Hue) Laundon | - | - | - | + |
| 39 | Lobothalia radiosa (Hoffm.) | - | - | + | - |
| 40 | Melanelixia fuliginosa (Fr. ex Duby) O. Blanco & al. | - | - | - | + |
| 41 | Myriolecis dispersa (Pers.) Śliwa, Zhao Xin & Lumbsch | - | - | - | + |
| 42 | Parmelia saxatilis (L.) Ach. | - | - | - | + |
| 43 | Parmelia sulcata Taylor | - | - | - | + |
| 44 | Peltigera didactyla (With.) Laundon | - | - | - | + |
| 45 | Phaeophyscia orbicularis (Neck.) Moberg | - | - | - | + |
| 46 | Phlyctis argena (Ach.) Flot. | - | - | - | + |
| 47 | Physcia ascendens (Fr.) H. Olivier | - | - | - | + |
| 48 | Physcia caesia (Hoffm.) Fürnrohr | - | - | - | + |
| 49 | Physcia dubia (Hoffm.) Lettau | - | - | - | + |
| 45 | Physconia grisea (Lam.) Poelt | - | - | - | + |
| 51 | Placynthiella icmalea (Ach.) Coppins & P. James | - | - | - | + |
| 52 | Polysporina lapponica (Schaer.) Degel. | - | - | - | + |
| 53 | Polysporina simplex (Davies) Vězda | - | - | - | + |
| 54 | Porina chlorotica (Ach.) Müll. Arg | - | - | - | + |
| 55 | Porpidia crustulata (Ach.) Hertel & Knoph | - | - | - | + |
| 56 | Porpidia tuberculosa (Sm.) Hertel & Knoph | - | - | - | + |
| 57 | Rhizocarpon distinctum Th. Fr. | - | - | - | + |
| 58 | Rhizocarpon geographicum (L.) DC. | - | - | - | + |
| 59 | Rhizocarpon grande (Flörke ex Flot.) Arnold | - | - | - | + |
| 60 | Rhizocarpon polycarpum (Hepp) Th. Fr. | - | - | + | - |
| 61 | Rhizocarpon reductum Th. Fr. | - | - | - | + |
| 62 | Rhizocarpon simillimum (Anzi) Lettau | - | - | + | - |
| 63 | Rinodina confragosa (Ach.) Körb. | - | - | - | + |
| 64 | Rinodina oleae Bagl. | - | - | - | + |
| 65 | Scoliciosporum umbrinum (Ach.) Arnold | - | - | - | + |
| 66 | Tephromela atra (Huds.) Hafellner | + | - | - | + |
| 67 | Tephromela grumosa (Pers.) Hafellner & Cl. Roux | - | - | - | + |
| 68 | Trapelia coarctata (Sm.) Choisy | - | - | - | + |
| 69 | Trapelia glebulosa (Sm.) J. R. Laundon | - | - | - | + |
| 70 | Trapelia obtegens (Th. Fr.) Hertel | - | - | - | + |
| 71 | Trapelia placodioides Coppins & P. James | - | - | - | + |
| 72 | Verrucaria muralis Ach. | - | - | - | + |
| 73 | Xanthoparmelia conspersa (Ach.) Hale | - | - | - | + |
| 74 | Xanthoparmelia loxodes (Nyl.) O. Blanco & al. | - | - | - | + |
| 75 | Xanthoria parietina (L.) Th. Fr. | - | - | - | + |
Lichen Biota State of Preservation
The viability of most lichens was assessed as good and very good in the examined localities. Most species have a crustose thallus, and the potential cause of the numerical predominance of lichens with this type of thallus in the studied area may be the young age of rock outcrops; this is because the foliose and fruticose lichens require more time to develop their thallus. The crustose thallus is less exposed to airborne contaminants than foliose or fruticose (Schöller, 1997), which might explain the small number of damaged lichen thalli found at the localities. Poor viability was determined for only two foliose species, Rusavskia elegans and Phaeophyscia nigricans, at locality No. 9; the thalli of these taxa did not reach their full sizes. Of note, individual specimens were observed, and may soon disappear from the locality. The observed abnormalities in the development of thalli might not be directly related to air pollution. The studied ecological areas are found in the forest (localities Nos. 7 and 10) or in its surroundings, on the edge of the village (locality No. 9). No sources of contamination were found near these areas. In locality No. 9, a municipal road that connects Przemiłów with other small villages exists. This road, although not frequently recurring, is a potential source of pollution. However, pollutant emissions do not have a significant impact on the other species at locality No. 9; thus, the cause of this condition must be different, causing these specimens to be extremely sensitive. The viability of the thallus of certain epilithic lichens may also be a result of the changing habitat conditions, and in particular, the progressive processes of secondary succession, manifested by the gradual overgrowth and overshadowing of the rock substrate by plants (Zalewska et al., 2004).
One of the most important factors influencing species diversity and biota preservation within the studied localities is the insolation. The light requirements of lichens vary by species; however, many lichens need sunlight and significant insolation (Gasulla et al., 2012) to develop properly and reach full size. An analysis of the results of the studies revealed a pattern based on the exposed rocks, which are more often hit by the sun’s rays, with more diversity and abundance of lichens observed. At locality No. 9, in Przemiłowo, an exposed quarry exists in the ecological area. In this open area without trees, which is full of sunlight, 32 species of lichen were found, thereby accounting for 68% of the total biota found. Therefore, the species can be concluded to mainly be heliophilous. Other ecological areas (locality Nos. 7 and 10) are found in the forest, under the treetops, whereas serpentinite rocks are often found in the immediate vicinity of grass, which serves as an additional shield from the sun’s rays. A markedly lower number of species were found at these localities. Interestingly, although light reaches both localities to a similar degree, the presence of the same species at localities Nos. 7 and 10 has rarely been reported.
To date, no studies have been conducted on the state of preservation of the lichen biota based on their frequency and viability in the ecological areas in the Ślęża Massif. In fact, only the discussed records of the species were established. The data collected and presented herein are complementary to those published on the lichen biota in the Ślęża Massif and serve as a basis for establishing protective measures and conducting further analyses.
Protection of Lichens in the Study Area
Preserving lichen biota in the ecological area through current and future protection, is of critical importance. Although most of the species found in the study localities are common in the country owing to significant transformation of habitats in Lower Silesia and the high level of pollution, every lichen-rich habitat is very valuable and should be monitored to preserve a high degree of biodiversity. Determining the degree of preservation of individual taxa may serve as a starting point for further research, and should involve the monitoring of the habitats presented in this study. The need to protect lichens in specific areas as outcrops of serpentinite rocks is additionally indicated by the unique and relatively rich nature of the biota in these areas compared to other regions in the Sudetes Foreland area, and the presence of species rarely seen, such as Caloplaca subpallida. For the overall preservation status of the lichen biota to remain good, some measures should be taken to prevent habitat changes that could adversely affect lichen health. The proposed active protection system, which can be used in the study area, involves the mowing of grass, which in many places overgrows rock outcrops, thereby serving as a huge threat, especially to ground species of the genus, Cladonia. Another action to protect lichen involves the prevention of tree and shrub expansion at locality No. 9, which is the most open and sunny; the excessive growth of trees and shrubs would cause the overshadowing of rocks and the extinction of heliophilous epilithic species. In addition, the increase in insolation, especially at locality No. 7, would favor the colonization of the previously overshadowed rock walls by more heliophilous species. Unfortunately, changing the light conditions could have a negative impact on Asplenium populations, which prefer humid and shaded habitats (Żołnierz, 2014a, 2014b, 2014c); therefore, it is difficult to implement in practice.
. Conclusions
Serpentine rock outcrops are very rare in Poland; however, the habitats associated with these types of rocks are extremely valuable and scientifically interesting.
The lichen biota in the area of the studied ecological localities in the Ślęża Massif is characteristic of serpentinite rocks; however, the overall state of its preservation can be described as moderate rather than good or very good. The preservation of most of the recorded taxa could be improved through adjustments of the light conditions in the studied localities. Due to the unique and valuable character of serpentinite rocks, as well as the impossibility of defining the preservation status of all noted lichen species, it is advisable to carry out further studies and to include other localities of the ecological areas for more comprehensive analyses.


