. Introduction
Macromycetes of the genus Pleurotus (Fr.) P. Kumm. are widely grown and consumed worldwide, especially in Europe and Asia. Pleurotus is highly nutritious, possesses medicinal characteristics, and are highly appreciated in the international cuisine for their organoleptic characteristics. According to Research and Markets (https://www.researchandmarkets.com/), “the mushroom cultivation market was estimated to account for a value of USD 16.7 billion in 2020.” The champignon – Agaricus bisporus (J. E. Lange) Imbach – stands out as the species with the highest global production (40%), followed by oyster mushrooms sensu lato (Pleurotus spp.) and shiitake, Lentinula edodes (Berk.) Pegler.
The oyster mushroom P. ostreatus (Jacq.) P. Kumm. is one of the most studied edible mushrooms because of its nutritional quality, ease of cultivation, and economic potential (Research and Markets, 2021). A very rich literature has never ceased to provide information on the cultivation of this species; the main parameters and protocols were summarized by Stamets and Chilton (1983) and updated by several authors (Mahari et al., 2020; Sánchez, 2010).
The cultivation of this product started in 1917–1919, but it has significantly developed in the sixties, mainly in Italy, former Czechoslovakia, and Hungary. Subsequently, it spread throughout the rest of Europe both for commercialization and self-consumption. Originally performed on trunks and straw, P. ostreatus cultivation has been increasingly focused on agricultural wastes and residues within a circular economy vision (Grimm & Wösten, 2018; Masevhe et al., 2016; Ozcariz-Fermoselle et al., 2019).
Biologically, P. ostreatus is a saprotroph species naturally occurring on several broadleaves. The pileus is cream colored to lead gray, 5–20 cm, typically ear-shaped or shell-shaped. Lamellae are grayish-whitish and decurrent. The stipe is eccentric, 2–3 cm long; smell and taste are pleasant and flesh is white and quite firm (Boccardo et al., 2008). Pleurotus ostreatus sensu stricto is regarded as worldwide distributed, although often in sympatry with other strictly related species, e.g., P. cornucopiae (Paulet) Rolland and other oyster mushrooms, e.g., P. eryngii (DC.) Quél. (Zervakis et al., 2014).
Regarding its nutritional quality, protein content from P. ostreatus is compared to that of meat, since 80% of P. ostreatus protein is digestible. In addition, P. ostreatus provides minerals (P, Fe, Ca, K), thiamine (vitamin B), riboflavin (vitamin B2), pyridoxine (B6), pantothenic acid, biotin, folic acid, nicotinamide, ascorbic acid (vitamin C), and ergosteine (provitamin D) (Bayona, 2012; La Guardia et al., 2005).
Since it is a saprotroph, P. ostreatus plays an important ecological role in the disintegration of organic waste because of its ability to degrade cellulose – the main component of the plant cell wall – an abundant material that is difficult to decompose because of its internal structure. This trophic strategy facilitates cultivation, as materials from the wild with similar residue composition can be used as a substrate (Hernández & López, 2006).
Stems and branches produced by sanitary pruning of deciduous fruit trees are considered a limitation for the production of these crops, as their inadequate management hinders activities such as weeding, phytosanitary applications, and fertilization. Furthermore, unmanaged residues are a potential source of pests and diseases. Edible fungi such as P. ostreatus, which are useful for degrading cellulose through the production of enzymes, can use semiwoody and lignocellulosic structures rich in ash, protein and fiber as substrates to grow.
In recent years, Colombia has remarkably increased the cultivation of edible mushrooms. The oyster mushroom has been regarded as the finest species to grow due to the traditional method of planting, rapid propagation, and cultivation with low requirements for technological inputs (Fernandez Uribe, 2014). Moreover, Colombia is a major producer of apples, peaches, and pears (particularly in the region of Boyacá); therefore, proper handling of pruning residues from trees represents a challenge for sustainable agriculture and a chance of production diversification for small farmers. Finally, biodegradation of spent mushroom compost in agroenvironments is particularly favored by Colombian climatic conditions and the richness of soil microbiota (Landínez-Torres et al., 2020; Landinez-Torres et al., 2019).
The aims of this study were: (i) to explore the potential of pruning residues from selected fruit trees as a substrate for the efficient cultivation of P. ostreatus, and (ii) to provide a user-friendly and scalable methodology for the cultivation of P. ostreatus by rural families.
. Material and Methods
Study Area
The field work was performed in the municipality of Nuevo Colón-Boyacá, on the FACA farm located at 05°21′ 45″ N and 73°27′ 39″ W, located at 2,561 m a.s.l, characterized by an annual average temperature of 15.1 °C, annual precipitation of 920 mm, and relative humidity of 66%.
Cultivation of the oyster mushroom P. ostreatus was performed at the experimental farm San Isidro Labrador, Soracá-Boyacá, belonging to the Juan de Castellanos University.
Experimental Design
Six novel cultivation substrates were tested; hay was assumed to be the absolute control, since it is a common and well-known substrate for P. ostreatus cultivation.
The treatments and absolute control consisted of four replicates. Substrates were set as follows (%w/w; based on wet weight): T1 – 100% hay; T2 – 100% apple tree chips; T3 – 100% peach tree chips; T4 – 100% pear tree chips; T5 – 50% hay + 50% apple tree chips; T6 – 50% hay + 50% peach tree chips; and T7 – 50% hay + 50% pear tree chips.
Field Work
Pruning residues from apple trees, Malus domestica (Suckow) Borkh., peach trees, Prunus persica (L.) Batsch, and pear trees, Pyrus communis L., from FACA farm and hay from local markets were mechanically milled to about 1 cm chips using GTM Professional GTS 1300 Wood Chipper (Brumar Srl; Asti, Italy).
Subsequently, wood chips and milled hay were separately sterilized in boiling water (approximately 80 °C due to altitude a.s.l.) for 1 hr. Part of the volume of water (75%) was poured into the sterilization container.
Further operations were not performed axenically.
Cultivation of Pleurotus ostreatus
Clear plastic bags (40 cm × 60 cm) were used for cultivation. Spawn of P. ostreatus provided by the National Training Service (Servicio Nacional de Aprendizaje, SENA) of Duitama, Boyacá (Colombia) was selected as the inoculum source.
Substrate layers (wood chips, hay, or a mixture of both) were alternated with spawn layers into the cultivation bags. The weight of 235 g per bag was set, with the inoculum being <12.5%. Cultivation bags containing the substrate and inoculum are hereafter referred to as breads.
Incubation was performed in a clean room at a relative humidity of 81% and an average temperature of 19.1 °C (thermo-hygrometer UNI-T reference UT 333; Uni-Trend Technology China). Breads were gently covered by a black geotextile to simulate dark conditions for 15–20 days after inoculation. When the primordia sprouted, the geotextile was removed, but direct lightening was avoided.
After primordia sprouting was observed, basidiomes were allowed to grow for 7–10 days before harvest. In parallel, a subsample of basidiomes was allowed to grow for 29 days to record the growth profile.
Bromatological Analysis
Bromatological analysis (ash, crude protein, ethereal extract, and crude fiber) was performed at the Animal Nutrition Laboratory of the Pedagogical and Technological University of Colombia (UPTC). Preliminary dehydration and measurement of dry weight were performed at the Plant Physiology Laboratory of the Juan de Castellanos University Foundation.
The analysis was performed in duplicates. Basidiomes of P. ostreatus from each treatment and hay were analyzed, as were wood chips from apple trees, peach trees, and pear trees.
For preliminary dehydration, samples were maintained in paper bags with the least amount of air possible, dried at a maximum temperature of 60 °C for 48 hr, and then processed in a mill with a 1-mm-diameter pore mesh.
The following methods were used for the bromatological analysis of P. ostreatus and the substrates used for production: the oxidation method (Helrich, 1990), crude protein with Kjeldahl (Association of Official Analytical Chemists, 1984), raw fiber with acid detergent (Horwitz, 2000), and ethereal extract with Soxlhet (Church et al., 2002). All analyses were performed according to the official laboratory protocols of the Animal Nutrition Laboratory of UPTC (Universidad Pedagógica y Tecnológica de Colombia).
Data Analysis
For the data analysis, the fulfillment of statistical assumptions (normality, homogeneity of variance, and independence) was verified. To determine significant statistical differences between treatments, the analysis of variance (ANOVA) was performed, followed by Tukey’s multiple comparison test with a significance level of 0.05. Data analysis was performed using RStudio statistical software (R 4.0.4 coupled with RStudio 1.4.1103 interface, RStudio, Boston, USA). Cluster analysis, Mann–Whitney U test, Kruskal–Wallis test, Dunnett’s T3 test, and Spearman’s ρ test were performed by IBM SPSS Statistics 21 (IBM, New York, USA). Other graphic designs were obtained using Microsoft Excel 2016.
. Results
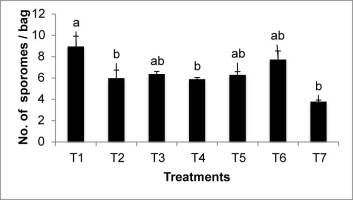
Regarding sprouting, estimated by the number of fingers obtained per bag, there were significant statistical differences confirmed by all the tests performed (Mann–Whitney U, Kruskal–Wallis, and Tukey’s). As shown in Figure 1, the treatments with the highest average number of sporomes were T1 (nine sporomes/bag), followed by T6 (eight sporomes/bag); T7 had the lowest number of sporomes (four sporomes/bag).
Sprouting was not correlated with any other morphological variables (pileus diameter, pileus thickness, and stipe length).
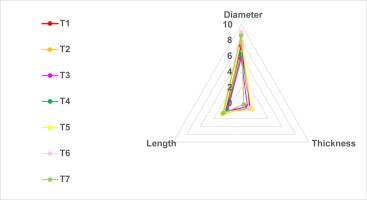
Morphometry relied on three variables: pileus diameter, pileus thickness, and stipe length. Significant statistical differences, confirmed by all the tests performed (Mann–Whitney U, Kruskal–Wallis, and Tukey’s), were found among treatments for all three variables.
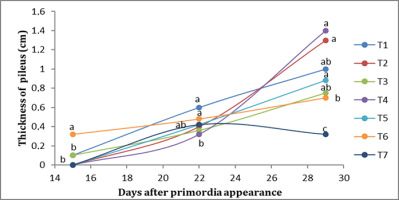
In the first week, T6 presented the greatest thickness of the pileus (0.32 cm), while T1 and T2 presented the lowest (0.10 cm). In the second week, T1 and T6 presented the greatest thickness of the pileus (0.50 cm), while T4 presented the lowest thickness of the pileus (0.32 cm). In the third week, T2 presented the highest thickness of the pileus (1.50 cm), followed by T5 (1.46 cm), while T7 presented the lowest thickness of the pileus (0.40 cm) (Figure 2).
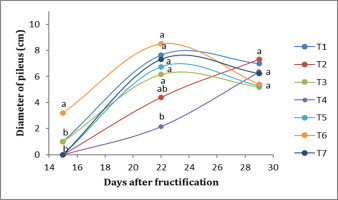
Regarding the diameter of the pileus, in the first week, T6 presented the greatest diameter of the pileus (3.2 cm), while the lowest values were obtained by T1 (1 cm), followed by T2 (<1 cm). In the second week, T6 presented the largest diameter (8 cm), followed by T1 (7.6 cm), while T4, on the other hand, presented the smallest diameter (2.15 cm). In the third week, there were no significant differences; T2 presented a diameter of the pileus of 7.8 cm, followed by T1, at 7 cm (Figure 3).
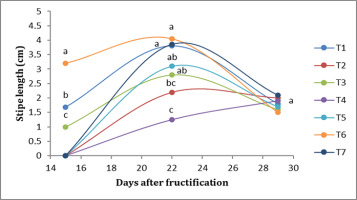
Regarding the length of the stipe, in the first week, T6 presented the longest stipe (3.20 cm), followed by T1 (1.68 cm), while T2 presented <1-cm stipes. In the second week, T6 treatment presented the longest stipe (3.85 cm), followed by T7 (3.56 cm); T2, on the other hand, presented the shortest length, at <1 cm. In the third week, there were no significant differences; T7 presented a stipe length of 2.12 cm, followed by T2, which presented a length of 2 cm (Figure 4).
According to the correlation analysis (Spearman’s ρ), a significant positive correlation was found between the diameter of the pileus and stipe length (ρ = 0.707, sig. H0 = 0.0%).
The morphology of sporomes grown on mixed substrates (hay + wood) was shifted toward higher diameters and stipe lengths than sporomes grown on pure wood or pure hay (Figure 5).
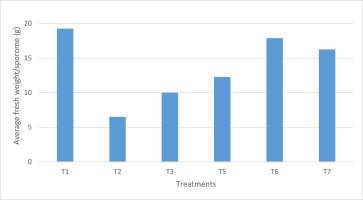
Regarding fresh weight, according to statistical analysis, T6 presented the highest fresh weight per sporome (27.2 g), followed by T1 (19.2 g), and T5 (16.25 g) (Figure 6).
Although the highest fresh weight values were found in T6 and T1, as well as the highest values of sporomes per bag, there was no significant correlation between average fresh weight per sporome and number of sporomes per bag.
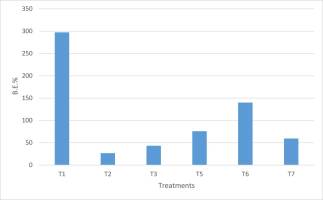
Biological efficiency (B.E.%), which is the % ratio between the cumulative fresh weight of mushrooms and dry weight of the substrate (Ahmed et al., 2009; Chang et al., 1981), was the highest in hay (Figure 7). Nevertheless, it should be noted that dry hay is a considerably lighter matter than wood. Thus, this result must not mask the values of B.E.% on other substrates, with particular concern to T6 (140%) and T5 (76%).
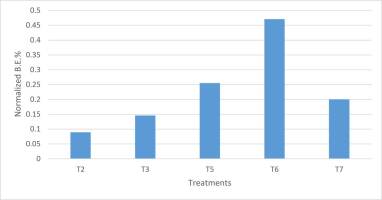
More interestingly, in Figure 8, the ratio between B.E.% in hay and other examined substrates (“normalized B.E.%” hereafter) is shown.
The highest normalized B.E.% was verified in T6; as a whole, mixed substrates recorded higher values than those of pure wood. Cluster analysis also highlighted this result (Figure 9).
Figure 9
Cluster analysis of normalized B.E.% by IBM SPSS 21. Clustering method: between groups linkage; measure by squared euclidean distance. Each substrate was treated as a variable.
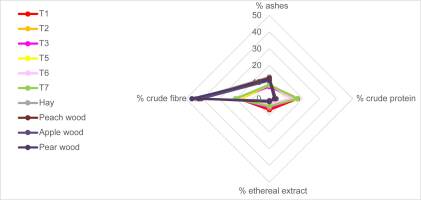
Bromatological analysis (Table 1) revealed similar comprehensive profiles in sporomes from different substrates according to Kruskal–Wallis test (p = 0.996). The bromatological profiles of pure substrates (hay and wood from different trees) were similar to each other (p = 0.962) as well. As expected, fungal profiles significantly differed from wood profiles, as they concern all the parameters: ash (p = 2.0%), crude protein (p = 2.0%), ethereal extract (p = 2.0%), and crude fiber (p = 2.0%). This can be easily visualized in Figure 10.
Table 1
Bromatological analysis of Pleurotus ostreatus from different substrates and analysis of pure wood.
Moreover, Figure 10 shows that wood profiles dramatically shifted towards high values of crude fiber; ashes were also higher in fungal sporomes than the other treatments. On the other hand, fungi showed an almost symmetric frame, which highlights the remarkable protein content.
Regarding the bromatological analysis of P. ostreatus, T1 presented the highest value in terms of crude protein (16.9%), followed by T7 (16.6%), and T2 and T6 (13.5% and 13.4%, respectively). The highest crude fiber value for P. ostreatus was found in treatments T7 (20.3%) and T3 (20.1%). Regarding the percentage of ethereal extract, it was observed that the treatments T1 (6.7%) and T2 (5.5%) stood out, presenting the highest levels. The highest concentration of ashes in the mushrooms was obtained in treatments T6 (8.6%) and T7 (8.4%).
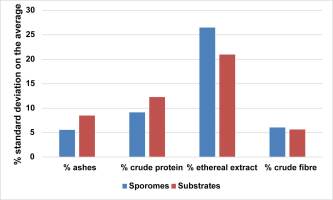
As a whole, each bromatological variable (ash, crude protein, ethereal extract, and crude fiber) showed no negligible intra-group variability (Figure 11). Thus, beyond the comprehensive bromatological profile, ethereal extract recorded the highest % standard deviation of the means in both sporomes and substrates. Regarding crude protein, the high % standard deviation of the means was due to the low values, which amplified the variation.
. Discussion and Conclusions
The sprouting results (number of sporomes per bag) can be attributed to the fact that the highest production of fungi is obtained in substrates rich in fiber and structural carbohydrates (Savón et al., 2007). Despite the fact that the fiber values were lower in hay than in other substrates examined in this study, the fiber of this material has a great ease of assimilation due to its physical constitution. In addition, hay is a well-known substrate for the cultivation of P. ostreatus (López-Rodríguez et al., 2008).
The morphometric results are consistent with the literature. As explained by Benavides (2013) and López-Rodríguez et al. (2008), the diameter of the pileus and stipe length depend on the maturity reached, as well as on the similarity of the size of the mushrooms.
According to Gaitán-Hernández et al. (2009), the diameter of the pileus, in particular, is more related to the genetic characteristics of the mushroom than to the type of substrate, since maintaining optimal environmental conditions for cultivation, the mushrooms will present homogeneity. Interestingly, mixed substrates (hay + wood) favored diametric and stipe growth in comparison to pure wood or pure hay.
B.E.% was similar to that reported in most studies (Familoni et al., 2018; Masevhe et al., 2016; Tekeste et al., 2020; Tisdale et al., 2006; Zervakis et al., 2013). Consistent with the literature, substrates composed of pure wood were less efficient than those composed of mixed hay-wood. Nevertheless, the present study reported a similar or higher B.E.% than that found by Jafarpour et al. (2010), who supplemented chips with more energetic materials than hay. Interestingly, in this study, B.E.% was higher than that reported by Siwulski et al. (2012), although the cultivation set was not with controlled lighting.
A part of the literature reports slightly higher values for crude protein than those found in the present study (Alam et al., 2008; Oyetayo & Ariyo, 2013; Patil et al., 2010). Nevertheless, it should be considered that quantitative analysis of total proteins is significantly affected by the method (Conklin-Brittain et al., 1999; Mæhre et al., 2018). This is also true for values obtained from substrates as well (Familoni et al., 2018). Interestingly, Wang et al. (2001) reported a very high % of crude protein but poor B.E.% on spent beer grains.
Analogously, the top crude fiber values (approximately 20%) from the present study are consistent with the literature (Alam et al., 2008; Oyetayo & Ariyo, 2013) or remarkably higher than those (Patil et al., 2010).
Ethereal extract is a difficult variable to compare with data reported in the literature, since it strongly depends on the method used (Alam et al., 2008; Oyetayo & Ariyo, 2013; Patil et al., 2010). In this study, ethereal extract and crude protein appeared as pivotal variables in the bromatological profile of P. ostreatus, since their variation was remarkable.
Unexpectedly, there was no significant correlation between the bromatological values of the substrate and sporomes. This is consistent with the results reported by Vega & Franco (2013), who argue that sporome development is substantially independent from the substrate when different nutrients are available, regardless of their proportion. According to Baena González (2005), ashes in P. ostreatus are strain-correlated instead of substrate-correlated.
On the other hand, Benavides et al. (2015) report a correlation between oil palm rachis in the substrate and ethereal extract of P. ostreatus sporomes. Conversely, according to Savón et al. (2007), substrate composition influences the quality and protein quantity of fungi, whereas ashes represent the mineral content that becomes available for fungal growth (Benavides, 2013).
Bromatological profiles of wood substrates are consistent with the literature (Familoni et al., 2018), although WingChing-Jones and Retana (2009) found lower content of crude fiber, and Jafarpour et al. (2010) found remarkably higher content of crude fiber than in other substrates. High fiber content is highly desirable; according to Hoyos et al. (2012), it is essential for the growth of P. ostreatus. Moreover, fibers can be incorporated into the soil as easily assimilated aggregates for crops.
In conclusion, pruning residues from apple trees, peach trees, and pear trees can be successfully used as raw materials to obtain substrates for P. ostreatus cultivation. Substrates composed of wood chips mixed with hay showed the best performance in terms of biological efficiency and morphometric parameters of sporomes. The 50% hay + 50% peach wood mix resulted in the highest values of those variables. Moreover, sprouting of primordia took a shorter time on this substrate than on the other substrates.
Therefore, the methodology applied in the present study is adequate for the production of P. ostreatus. The cultivation protocol was confirmed to be user-friendly for scalable production by small farmers. Substrate disinfection is a fundamental factor in the reduction of microbial competitors; moreover, heat disinfection partially alters substrate consistency and favors fungal colonization.