. Introduction
Natural disturbances (e.g., fires, flooding, windstorms, and insect outbreaks), especially large-area canopy damage, play a crucial role in forest dynamics, inter alia, as an essential driver of biological diversity. Moreover, the success of the demographic expansion of a wide range of species from all taxonomic groups is strongly dependent on natural disturbances (e.g., Dobrowolska, 2010; Pickett & White, 1985; Willig & Presley, 2018). However, knowledge about the role of disturbances in the regeneration of forest ecosystems is still incomplete (Obidziński, 2001; Szwagrzyk, 2000; Szwagrzyk et al., 2018). The importance of disturbances for biodiversity, including species diversity, was confirmed by studies of mycobiota in the areas damaged by fire, where many species new to Kampinos National Park (KNP) in central Poland were found (Gierczyk et al., 2017; Gierczyk, Szczepkowski, Kujawa, & Ślusarczyk, 2019; Gierczyk, Szczepkowski, Ślusarczyk, & Kujawa, 2019). Studies of various groups of spore organisms (fungi, lichens, and bryophytes) on windthrown trees have provided new data on the diversity and ecology of these organisms in KNP (Zaniewski & Fojcik, 2020; Zaniewski et al., 2019).
The occurrence of strong winds in Poland has been increasing in recent decades (Lorenc, 2012). Windstorms have caused numerous disturbances and catastrophic destruction of managed forest stands (e.g., Chojnacka-Ożga & Ożga, 2018a; Taszarek & Gromadzki, 2017; Zajączkowski, 1991). Disturbances of forests also occurred a few times in KNP due to strong winds, for example, in 1964, 1972, 1980, 1993, 1994, 2004, and 2017 (Andrzejewska, 2003; Hryniewiecki, 2004; Lorenc, 2012; Lubański, 2003; Tyburski, 2015, 2019; Tyburski & Przybylski, 2016). Recently, two strong winds have damaged a few fragments of forests in the KNP at the beginning of the summer of 2017. This windthrow incident enabled a study on postdisturbance mycobiota of the Park, with particular emphasis on the fungi occurring on different parts of windthrown trees. In the first year of study (2018), 62 species of fungi were found on 30 trees belonging to three genera (Betula pendula, Pinus sylvestris, Quercus petreae, Q. × rosacea) (Zaniewski et al., 2019).
The aim of this paper is to present the species of fungi new to KNP found in the areas of the windthrow during the first two years of study (2018–2019). Additionally, one interesting species of fungus new to the Park buffer zone has been included.
. Material and Methods
Location and Description of Research Plots
The study was carried out on two plots located in the Rózin and Grabina Protective Subdistricts/Forest Subdistricts (in Kampinos Protective District/Forest District) (Figure 1). The Grabina plot is located in the partial protection area in forest compartments No. 125a and 125c. The 84-year-old damaged stand was dominated by Scots pine Pinus sylvestris with singly-occurring common aspen Populus tremula and two birch species: silver birch Betula pendula and downy birch B. pubescens (Figure 2). As a result of the windfall, a canopy gap (0.6 ha) of an almost circular shape was created, with a completely damaged stand. The trees in the damaged pine stand were planted on ridges and deep furrows, which are still visible today (Forest ecosystem protection operate, 2002).
The Rózin plot is also located in the partial protection area in forest compartments No. 258a and 258b. The damaged stand was approximately 104 years old and comprised planted trees. The stand consisted of a 90% share of two oak taxa: sessile oak Quercus petraea and Q. × rosacea with a 10% share of silver birch and sparse Scots pine trees (Figure 3) (Forest ecosystem protection operate, 2002). The windfall plot occupies an area of approximately 5 ha in the shape of an irregular and broken streak. The stand was only partially destroyed. The examined oaks belonged to the sessile oak species or represented hybrids of sessile oak and pedunculate oak (Q. robur), characterized by a predominance of sessile oak traits, i.e., Q. × rosacea.
Methods
Field studies were conducted between May and October 2018 and 2019. Thirty windthrown trees, with 10 trees of each species (oak, birch, and pine), were subjected to a detailed study on windfall plots. Fungal species composition was investigated in 12 parts of each tree comprising tree pit, soil of lower side root disc, roots of lower side root disc, soil of upper root disc, roots of upper root disc, trunk bottom (0–1 m high), lower trunk (1 m – half of the trunk height), upper trunk (half of the trunk height – base of the crown), thick branches of lower crown, thin branches of lower crown, thick branches of upper crown, and thin branches of upper crown. Branches up to approximately 7 cm in diameter were considered thin, and those higher than this value as thick. The collected specimens were identified using standard mycotaxonomical methods (Clemençon, 2009). Specimens were identified using the following monographs: Daldinia (Stadler et al., 2014), Mycena, Phloeomana (Aronsen & Læssøe, 2016), pyrenomycetes (Ellis & Ellis, 1997; Wergen, 2018), Odonticium, Phanerochaete, Punctularia (Bernicchia & Gorión, 2010), Ganoderma, Spongipellis, and Trichaptum (Bernicchia, 2005; Ryvarden et al., 2017; Tomšovský, 2012). The nomenclature of basidiomycetous fungi was used according to Funga Nordica (Knudsen & Vesterholt, 2012) and the above-mentioned monographs and for ascomycetes according to MycoBank Database (http://www.mycobank.org/). Plant names are according to Mirek et al. (2002). Threat categories of fungal species in Poland are according to the “Red list of the macrofungi in Poland” (RL) (Wojewoda & Ławrynowicz, 2006). Forest compartment numbers were obtained from the Forest Data Bank (https://www.bdl.lasy.gov.pl/). Dried specimens were deposited in the fungarium of the Department Forest Protection of the Warsaw University of Life Sciences – SGGW (WAML) and the private fungaria of B. Gierczyk (BGF) and T. Ślusarczyk (TSH). Each number represents a different collection.
. Results – List of the Species
In the course of the study, a total of 185 taxa were collected (identified to the level of species, forms, varieties, and – in a few cases, of genera) (unpubl. data), including 18 species new to the KNP and its buffer zone, which are presented below.
Abbreviations: AK – Anna Kujawa; ASz – Andrzej Szczepkowski; BG – Błażej Gierczyk, TŚ – Tomasz Ślusarczyk; FD – forest district, KNP – Kampinos National Park, LP – landscape park, NP – national park; PD – protective district; PSD – protective sub-district; res. – nature reserve; RL – red-listed species (threat categories: R – rare; V – vulnerable; I – indeterminate).
Ascomycota
Ciliolarinacfr.laricina (Raitv.) Svrček. Specimen examined: KNP, Łubiec, 2.5 km NW, Leszno municipality, Kampinos PD, Grabina PSD, forest compartment No.: 125a,c; 2019-10-27; numerous ascomata on the trunk of the fallen Pinus sylvestris; leg. & det. TŚ; TSH 509/2019. Notes: Saprobic species inhabiting twigs, branches, bark, and wood of Larix, Pinus, and Picea. In Poland, it was reported from Lasy Janowskie LP (Chmiel, 1997) and Wielkopolska region (Gierczyk & Ślusarczyk, 2020). Studied specimens are most similar to C. laricina except for dimensions of spores (6–8 × 2.5–3 µm in the former in comparison to 7–11 × 2.5–3.5 µm in the latter). Some other Ciliolarina species occurring on pine wood: C. pinicola (Henning & Plöttner) Huhtinen (differing in colored excipular cells, apical pore not blue in Melzer reagent, and longer as well broader spores), C. neglecta Huhtinen (differing in short stipitate apothecia, and shorter as well narrower spores) (Huhtinen, 1993; Raitviir, 2004).
Daldinia petriniae Y. M. Ju, J. D. Rogers & F. San Martín (Figure 4). Specimen examined: KNP, Zaborówek, 1.5 km NNW, Leszno municipality, Kampinos PD, Rózin PSD, forest compartment No.: 258a, b; 2018-09-10, 2019-08-31; numerous stromata on branches of the fallen Betula pendula; leg. & det. TŚ, ASz, BG; TŚF282/2018, WAML 1041, 1051, BGF0004280. Notes: It is a rare species, growing on Betulaceae, mainly on Alnus and Betula. In Poland, D. petriniae has been reported from Starożyn res. (Stadler et al., 2014) and Gorce Mts (Wojewoda et al., 2016). It has also been collected in Las Marceliński in Poznań, BGF0004768 (Gierczyk, unpub. data). It is probably more common and widespread but may be confused with other Daldinia species.
Figure 4
Stromata of Daldinia petriniae in the Kampinos National Park; August 31, 2019. Photography by A. Kujawa.

Dendrostoma leiphaemia (Fr.) Senan. & K. D. Hyde. (Figure 5). Specimen examined: KNP, Zaborówek, 1.5 km NNW, Leszno municipality, Kampinos PD, Rózin PSD, forest compartment No.: 258a, b; 2018-09-10; numerous pseudostromata on dead twigs of the fallen Quercus petraea; leg. & det. ASz; WAML1043. Notes: It is common species in Poland (Mułenko et al., 2008). The frequent association of Dendrostoma spp. with Cytospora spp. may suggest weak or optional parasitism (Jaklitsch & Voglmayr, 2019).
Figure 5
Pseudostromata of Dendrostoma leiphaemia from the Kampinos National Park; September 9, 2018. Photography by A. Szczepkowski.

Diatrypella favacea (Fr.) Ces. & De Not. Specimen examined: KNP, Zaborówek, 1.5 km NNW, Leszno municipality, Kampinos PD, Rózin PSD, forest compartment No.: 258a,b; 2018-09-10, 2019-06-01; numerous stromata on branches of the fallen Betula pendula; leg. & det. TŚ; TŚF 508/2019. Notes: Saprobic species growing on twigs and branches of various deciduous trees. There are over 20 localities of this species in Poland, and it seems common here (Kujawa, 2020; Mułenko et al., 2008).
Nemania serpens (Pers.) Gray. Specimen examined: KNP, Zaborówek, 1.5 km NNW, Leszno municipality, Kampinos PD, Rózin PSD, forest compartment No.: 258a,b; 2018-09-10; numerous stromata on branches of the fallen Quercus petraea; leg. & det. TŚ; TŚF285/2018. Notes: Saprobic species inhabiting wood of many deciduous trees. In Poland, it is known from over 20 historical and contemporary localities (Kujawa, 2020; Mułenko et al., 2008).
Pseudovalsa umbonata (Tul.) Sacc. Specimen examined: KNP, Zaborówek, 1.5 km NNW, Leszno municipality, Kampinos PD, Rózin PSD, forest compartment No.: 258a,b; 2019-06-01; a dozen stromata on branches of the fallen Quercus petraea; leg. & det. TŚ; TŚF 510/2019. Notes: It is inhabiting twigs and branches of deciduous trees. In Poland, there are historical records from Dolny Śląsk region (Schroeter, 1908) and Białowieża Primeval Forest (Truszkowska, 1965, 1976).
Rosellinia aquila (Fr.) De Not. (Figure 6). Specimen examined: KNP, Zaborówek, 1.5 km NNW, Leszno municipality, Kampinos PD, Rózin PSD, forest compartment No.: 258a,b; 2019-06-01, 2019-08-31; numerous of stromata on branches of the fallen Quercus petraea; leg. & det. BG, ASz; BGF0004084, WAML 1044, 1045. Notes: It is quite common in Poland and has been reported from bark of various trees. Mentioned from several historical locations from Dolny Śląsk and Śląsk Opolski regions (Schroeter, 1908, as R. byssiseda). Later reported from Puszcza Śnieżnej Białki res. (Truszkowska, 1977), Strzelin hills (Truszkowska & Chlebicki, 1983), Babia Góra (Bujakiewicz, 2004, 2018; Chlebicki, 1989, 2018), Bieszczady Mts (Domański et al., 1960), Białowieża NP (Chlebicki et al., 1996; Faliński & Mułenko, 1997), Tatry NP (Mułenko et al., 2004; Scheuer & Chlebicki, 1997), and Puszcza Drawska (Kwaśna & Łakomy, 2006).
Figure 6
Stromata of Rosellinia aquila in the Kampinos Park; August 31, 2019. Photography by A. Szczepkowski.

Sarea resinae (Fr.) Kuntze. Specimen examined: KNP, Łubiec, 2.5 km NW, Leszno municipality, Kampinos PD, Grabina PSD, forest compartment No.: 125a,c; 2019-09-01; a few ascomata on a trunk of the fallen Pinus sylvestris; leg. & det. ASz; WAML 1032. Notes: It is a resinicolous fungus. It was previously faultily considered as a species of lichens and, therefore, often listed with them (Beimforde et al., 2020). This saprobic fungus is rather common in Poland but probably overlooked.
In Poland, it is known from (i) a few historical records, e.g., Stadtwald pr. Angerburg (forest area E of Węgorzewo), Skomantberg pr. Oletzko (Skomentno Wielkie, near the Skomentno Lake, Olecko), Ostrokollen (Ostrykół near Ełk), Kopyken pr. Lyck (Kopijki near Ełk) (Lettau, 1912, after Ohlert, 1870, as Tromera resinae Fr.; Ohlert, 1870, as Lecidea resinae Fr.), Babia Góra (Rehman, 1879), Góry Świętokrzyskie (Błoński, 1890, as Tromera resinae Kbr.) and (ii) a dozen contemporary records, e.g., Buki Mierzei Wiślanej res. [Kowalewska & Kukwa, 2013, as Pycnidiella resinae (Fr.) Höhnel], Wdzycki LP (Kukwa et al., 2012, as P. resinae), Drawieński NP (Schiefelbein et al., 2012), Special Area of Conservation Natura 2000 “Swajnie” in Wichrowo FD (Szymczyk et al., 2014), Suwalski LP (Jando & Kukwa, 2003, as P. resinae), Starożyn res. and Mały Borek res. in Puszcza Augustowska (Czyżewska et al., 2005), Białowieża NP (Kukwa et al., 2008), Górny Śląsk region including Świerklaniec FD (Kowalski, 1990; Kowalski & Domański, 1983; Kowalski et al., 1994, as P. resinae). Recently, its teleomorphic stage was found on Larix decidua in Suchedniów FD (WAML 951), on Larix decidua var. polonica in Dukla FD (Modrzyna res.) (WAML 862), on Larix sp. in Rogów FD (Forest Experimental Station of the Warsaw University of Life Sciences – SGGW in Rogów) (WAML 856), on Picea abies in Pisz FD, and Białowieża FD (WAML 861) (Szczepkowski, unpbl. data).
Sphaeropsis sapinea (Fr.) Dyko & B. Sutton. Specimen examined: KNP, Łubiec, 2.5 km NW, Leszno municipality, Kampinos PD, Grabina PSD, forest compartment No.: 125a,c; 2018-07-21; numerous of pycnidia on shoot of the fallen Pinus sylvestris; leg. & det. ASz; WAML 1042. Notes: It is an opportunistic pathogen that causes a widespread disease known as Sphaeropsis blight (Diplodia tip blight or shoot dieback of conifers). In Poland’s forests, S. sapinea is probably a common species but it is not reported often (Sierota et al., 2019). It is hitherto mentioned from a dozen forest districts e.g. Brynek FD (Kowalski, 1988; Kowalski & Poździk, 1993), Chrzanów FD (Kowalski, 1988), Herby FD (Kowalski, 1988), Leżajsk FD (Kowalski, 1988), Miechów FD (Kowalski & Zych, 2002b), in the “Puszcza Białowieska” Promotional Forest Complex (Kowalski et al., 2019), Świerklaniec FD (Kowalski, 1988; Kowalski & Zych, 2002a, 2002b), some FD of the Lublin Regional Directorate of the State Forests (Król et al., 2015), area of the Toruń Regional Directorate of the State Forests (Stocka, 2010), three national parks: Białowieski (Faliński & Mułenko, 1997), Wielkopolski (Kartawik et al., 2019), and Wigierski (Baturo-Cieśniewska et al., 2020), Kórnik Arboretum (Przybył, 1990). It has also been collected in Browsk FD (WAML 1052), Hajnówka FD, Skrwilno FD (WAML 1066), Rogów FD (Forest Experimental Station of the Warsaw University of Life Sciences – SGGW in Rogów) (WAML 1061), and in green areas of Ciechanów on Pinus mugo and P. nigra (WAML 1064) and in Warsaw on P. sylvestrisand P. nigra (WAML 1065) (Szczepkowski, unpbl. data).
Tubeufia cerea (Berk. & M. A. Curtis) Höhn. Specimen examined: KNP, Zaborówek, 1.5 km NNW, Leszno municipality, Kampinos PD, Rózin PSD, forest compartment No.: 258b; 2018-09-10; over a dozen perithecia on stromata of probably Diatrypella sp. on the fallen twig of Quercus petraea; leg. ASz, det. TŚ; TŚF 316/2018. Notes: It is growing on stromata of pyrenomycetous fungi. In Poland, it was reported from vicinity of Toruń (Weber-Czerwińska, 1967), Babia Góra (Chlebicki, 1989), Białowieża NP (Chlebicki & Skirgiełło, 1995; Faliński & Mułenko, 1997), and Puszcza Romincka (Chlebicki, 2005; Chlebicki & Skirgiełło, 1995).
Basidiomycota
Ganoderma adspersum (Schulzer) Donk (Figure 7). Specimen examined: KNP buffer zone, in Laski village at 3 Maja Str., near the church; 2019-11-09; three basidiomata in the lower part of the trunk of dying Aesculus hippocastanum; leg. & det. ASz; WAML 1046. Notes: Parasite or saprobic species inhabiting mainly deciduous trees. It is rather common in Poland, known from over 30 localities (Kujawa, 2020; Sokół, 2000; Wojewoda, 2003).
Figure 7
Basidioma of Ganoderma adspersum from Laski village in the Kampinos National Park buffer zone; November 11, 2019. Photography by A. Szczepkowski.

Mycena hiemalis (Osbeck) Quél. Specimen examined: KNP, Zaborówek, 1.5 km NNW, Leszno municipality, Kampinos PD, Rózin PSD, forest compartment No.: 258a,b; 2019-10-26; a few basidiomata on the upper surface of the rootball of fallen Quercus petraea and Betula pendula; vid. BG. Notes: Species common in Poland, known from over 20 localities (Kujawa, 2020; Wojewoda, 2003).
Mycena smithiana Kühner. Specimen examined: KNP, Łubiec, 2.5 km NW, Leszno municipality, Kampinos PD, Grabina PSD, forest compartment No.: 125a,c; 2019-10-27; numerous basidiomata on decaying Quercus sp. leaves in pine pit; leg. & det. BG; BGF0004669. Notes: It is quite common, saprobic species growing exclusively on fallen oak leaves. In Poland, hitherto mentioned from numerous localities, although only in few reports leaves or litter are listed as substrate, i.e., Dolina Rzeki Brdy res. (Ławrynowicz et al., 2002a, 2002b, as M. debilis), Łaznów res. (Szkodzik, 2005, as M. debilis), Karkonosze NP (Lisiewska, 1992, as M. debilis), the Świętkorzyskie Mts (Łuszczyński, 2007, 2008), the vicinity of Czerniejewo (Lisiewska, 1987), and historical locality in Radków (Schroeter, 1889). It has been also mentioned from Bielinek res. [Bujakiewicz, 1997, as M. debilis (Fr.) Quél.], Buki nad Jeziorem Lutomskim res. (Bujakiewicz & Springer, 2009), Jelonka res. (Kałucka, 2009), Trębaczew res. (Ławrynowicz, 1973, as M. debilis), Wielka Kępa Ostromecka res. (Bujakiewicz, 1992, as M. debilis) and Babia Góra NP (Bujakiewicz, 1979, 2004, 2018, as M. debilis); however, the substrata mentioned in these reports (wood, juniper needles) indicate incorrect determination.
Figure 8
Basidiomata of Odonticium septocystidiatum from the Kampinos National Park. August 31, 2019. Photography by B. Gierczyk.

Odonticium septocystidiatum (Burt) Zmitr. & Spirin (Figure 8, Figure 9). Specimen examined: KNP, Zaborówek, 1.5 km NNW, Leszno municipality, Kampinos PD, Rózin PSD, forest compartment No.: 258b; 2019-08-31; few basidiomata on the trunk of the fallen Quercus sp. (?); leg. & det. BG; BGF0004282. Notes: Species hitherto not reported from Poland. In Europe, it is not rare and known from Belgium, the Czech Republic, Denmark, Estonia, Finland, France, Germany, the Netherlands, Norway, Portugal, Russia, Slovakia, Spain, Sweden, Switzerland, and the United Kingdom (Antonín et al., 2006; Bernicchia & Gorjón, 2010; Borges et al., 2010; Dingemans, 2021; Dämon, 2001; Kučera & Kautmanová, 2011; “Odonticium septocystidiatum,” 2021; Volobuev & Tokareva, 2017). Odonticium septocystidiatum grows on wood and bark of various coniferous and deciduous trees. Its septate, encrusted, cylindrical cystidia, and absence of clamp connections make it easy to recognize. Similar septocystidia are present in the hymenium of Ceraceomyces eludens, Hyphoderma setigerum, Hyphodontia ssp., and Suillosporium cystidiatum; however, these taxa differ by the presence of clamps and spore shape and size. Species description: Basidiomata resupinate, smooth to slightly tuberculate, dark cream-colored to orange. Margin paler, indistinctly fibrillose. Hyphal system monomitic. Context hyphae thin-walled; subiculum composed of thick-walled elements. Crystalline and amorphic grains present in hymenium and subiculum. Basidia four-spored, clavate. Spores allantoid, hyaline, smooth, without iodine reaction, 4.5–6 × 1.5–2.5 µm. Cystidia (septocystidia) projecting, up to 140 µm long, cylindrical, septate, composed of five–eight short-celled elements, encrusted with yellowish resinous grains. Clamps absent.
Figure 9
Microcharacters of Odonticium septocystidiatum: (A) basidioma cross section; (B) spores. Scale bars: 10 µm. Drawing by B. Gierczyk.
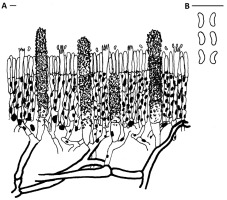
Figure 10
Basidiomata of Punctularia strigosozonata from the Kampinos National Park; September 1, 2019. Photography by A. Szczepkowski.

Phanerochaete magnoliae (Berk. & M. A. Curtis) Burds. Specimen examined: KNP, Zaborówek, 1.5 km NNW, Leszno municipality, Kampinos PD, Rózin PSD, forest compartment No.: 258a,b; 2019-10-26; a few basidiomata on branches of the fallen Betula pendula; leg. & det. TŚ; TŚF 511/2019. Notes: Saprobic species inhabiting wood of deciduous trees. In Poland reported from the Beskid Sądecki Mts (Wojewoda, 2003), Kaszubski LP (Karasiński, 2016), and the Gorce Mts (Wojewoda et al., 2016).
Punctularia strigosozonata (Schwein.) P. H. B. Talbot; (Figure 10); RL-E. Specimen examined: KNP, Łubiec, 2.5 km NW, Leszno municipality, Kampinos PD, Grabina PSD, forest compartment No.: 125a,c; 2019-09-01, 2019-10-27; a few basidiomata on trunk of the fallen Populus tremula; leg. & det. ASz, BG; WAML 1033, 1040, BG 0004688. Notes: Very rare species, in Poland hitherto reported from Międzyrzec Podlaski (Bresadola, 1903, as Phlebia rubiginosa Berk. et Rav.), Białowieża Primeval Forest (Gierczyk et al., 2013, 2014; Gierczyk, Ślusarczyk, et al., 2019; Kujawa et al., 2018; Szczepkowski et al., 2010; Wojewoda, 2002), Starożyn res. located in Puszcza Augustowska (Wojewoda, 2000, 2002) and Puszcza Knyszyńska (Kujawa et al., 2019).
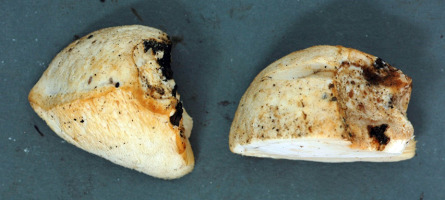
Spongipellis litschaueri Lohwag (Figure 11). Specimen examined: KNP, Zaborówek, 1.5 km NNW from, Leszno municipality, Kampinos PD, Rózin PSD, forest compartment No.: 258b; 2018-09-10; one basidioma on the trunk of the fallen Quercus petraea; leg. & det. BG; BGF0002803. Notes: Very rare species, in Poland hitherto known from Białowieża Primeval Forest (Karasiński & Wołkowycki, 2015) and Osetno res. (Karasiński & Domian, 2016). It grows predominantly on living or dead Quercus. It has been synonymized with S. delectans (Peck) Murrill; however, a molecular analysis confirmed that they are separate species (Tomšovský, 2012).
Figure 11
Cross-sectioned basidioma of Spongipellis litschaueri from the Kampinos National Park; September 10, 2018. Photography by B. Gierczyk.
Trichaptum biforme (Fr.) Ryvarden; RL-R. Specimen examined: KNP, 1.5 km NNW from Zaborówek, Leszno municipality, Kampinos PD, Rózin PSD, forest compartment No.: 258a, b; 2019-08-31, 2019-10-26; numerous of basidiomata on a trunk of the Quercus petraea and Betula pendula; leg. & det. ASz; WAML 1047, 1048, 1049. Notes: This species was observed by authors also in forest compartment No. 76h in 2017–2018. Species common in Poland, it is known from over 30 localities (Kujawa, 2020; Wojewoda, 2003; Wojewoda et al., 2002).
. Discussion
In Poland, strong winds and even hurricanes have disturbed forest ecosystems on numerous occasions in recent years (Chojnacka-Ożga & Ożga, 2018a, 2018b; Sierota et al., 2019; Taszarek & Gromadzki, 2017). However, no mycological or phytopathological studies have been conducted in such areas in the country. Field research in KNP in the years 2018–2019 resulted in interesting mycological findings with 18 novel taxa for KNP: 10 Ascomycota and eight Basidiomycota. One species (Ganoderma adspersum) was found on a dying horse chestnut tree in the Park buffer zone. Seventeen of them were found during the studies on wind-damaged areas after the gale, which took place in 2017. Among them, one species (Odonticium septocystidiatum) has not been reported previously from Poland. Some have been recorded very rarely in Poland, hitherto known from a few localities (Ciliolarina cfr. laricina, Daldinia petriniae, Pseudovalsa umbonata, and Spongipellis litschaueri). Two species, Punctularia strigosozonata and Trichaptum biforme, are placed in endangered and rare threat categories in the “Red list of the macrofungi in Poland,” respectively (Wojewoda & Ławrynowicz, 2006). While the former species is very rare and threatened in Poland, the second one is known from many localities (Kujawa, 2020); we recommend that it should not be included in the “Red list of the macrofungi.” Seven species new to KNP were found on windthrow-oaks (Dendrostoma leiphaemia, Mycena hiemalis, Nemania serpens, P. umbonata, Rosellinia aquila, Tubeufia cerea, and Trichaptum biforme), five on windthrow-birches (D. petriniae, Diatrypella favacea, M. hiemalis, Phanerochaete magnoliae, and T. biforme), four on windthrow-pines (Ciliolarina cfr. laricina, Mycena smithiana, Sarea resinae, and Sphaeropsis sapinea), and one on windthrow-aspen (P. strigosozonata). One of the listed species (Sarea resinae) was recently found on a spruce trunk in the wind-destroyed forest stands of “Szast” Protective Forest (Pisz FD) (Szczepkowski, unpbl. data). Some of the species (e.g., M. smithiana, S. sapinea, and T. biforme) have also been found in forests damaged by fire, industrial emissions, and other factors (Kałucka, 2009; Kowalski, 1988; Kowalski & Zych, 2002a, 2002b). In contrast, they have also been reported from well-preserved forests and managed forests without large natural disturbances. Eight ascomycetes (Ciliolarina cfr. laricina, Daldinia petriniae, Dendrostoma leiphaemia, Diatrypella favacea, N. serpens, P. umbonata, R. aquila, and S. sapinea) and eight basidiomycetous (G. adspersum, Mycena hiemalis, M. smithiana, O. septocystidiatum, Phanerochate magnoliaae, Punctularia strigosozonata, S. litschaueri, and T. biforme) fungi new to KNP are saprophytes or occasional pathogens. They were associated with branches and trunks of windthrow trees and other woody remnants. Fallen twigs and small branches are typically dominated by Ascomycota and corticoid Basidiomycota, which may develop from latent propagules (Boddy & Heilmann-Clausen, 2008). One species, G. adspersum, was found on the trunk of a dying tree in the Park buffer zone. Moreover, one species collected (S. resinae) is resinicolous fungus species and another species (T. cerea) is growing on stromata of pyrenomycetous fungi. The last species was also reported on wood and bark lying on the ground and on herbaceous substrates, but it could possibly grow on the mycelium of other ascomycetes (Sánchez & Bianchinotti, 2010).
The current results increase the number of taxa reported from KNP (without including phytopathogenic microfungi) to 1,630 (1,408 Basidiomycota and 222 Ascomycota) (Gierczyk, Szczepkowski, Ślusarczyk, & Kujawa, 2019; Marciszewska et al., 2020). The studies on wind-damaged areas of KNP will be continued in the coming years, and the results and analyses of the succession of fungal biota will be published.