Introduction
The Wielkopolski National Park (Polish: Wielkopolski Park Narodowy; WPN) is a lowland nature protection area in western Poland, ca. 15 km SW from Poznań City. The area of the WPN is composed of one major fragment bordering with the Warta River and divided by two major roads: a national road, DK5, crossing the park between the villages of Szreniawa and Stęszew, and a provincial road, No. 430, between the villages of Luboń and Mosina, and a few exclaves near Wielkawieś and Długosławiec and within Puszczykowo villages. The WPN is surrounded by several large villages (e.g., Luboń, Mosina, Puszczykowo, and Stęszew) and many small ones; several small settlements are also located within the Park area (Figure 1). The WPN was established in 1957 and enlarged in 1996. The total area of the WPN is 7,619.8 ha, while its buffer zone is 7,383.2 ha. The WPN is located in the Wielkopolskie Lakeland (Polish: Pojezierze Wielkopolskie) macroregion, in the mesoregions of Grodzisk Heights (Polish: Wysoczyzna Grodziska), Śrem Basin (Polish: Kotlina Śremska), and Poznań Gap of the Warta (Polish: Poznański Przełom Warty) (Solon et al.,2018). The whole area of the WPN is a part of the Ostoja Wielkopolska Natura 2000 protected area. The terrain sculpture of the WPN was formed by a glacier during the Weichselian glaciation; the major part of the Park area is ground moraine height, furrowed by tunnel valleys (Bartkowski,1972; Krygowski,2007). The watercourse network of the WPN is scanty, limited to the Samica and Wirynka rivers, and a few nameless streams. Much richer is the system of standing water, composed of several ribbon lakes (Figure 2), small, more or less circular lakes located in the deepest parts of the tunnel valleys and some periodic reservoirs. The soils of the WPN are mostly of postglacial origin, formed of gravels, sands, and clays, mainly brown leached soils and podzolic soils (Borowiec,1971,1973). Richer soils are present in dips of the land (black soils, muck soils, or peat soils) (Borowiec,1973). The WPN area is dominated by forest ecosystems. The most important trees occurring in the WPN are Pinus sylvestris, Acer platanoides, A. pseudoplatanus, Alnus glutinosa, Betula pendula, Carpinus betulus, Fagus sylvatica, Populus tremula, Quercus petraea, Q. robur, Q. rubra, and Tilia cordata (Żukowski et al.,1995). On the maps of potential natural vegetation, the dominating forest type in the WPN is Gallio-Carpinetum, while the other types are Salici-Populetum, Querco-Pinetum, Calamagostrio-Quercetum, Potentillo albae-Quercetum typicum, Fraxino-Alnetum, Leucobryo-Pinetum, and Ficario-Ulmetum chrysosplenietosum (Matuszkiewicz,2008). In fact, most of the WPN area is covered with substitutive forest communities with the domination of Pinus sylvestris, Quercus spp., and Carpinus betulus. Nonforest areas occurring in the Park are either (semi)natural (grasslands, meadows, fens, and reed beds) or anthropogenic communities (fields, pastures, road margins, gardens, and parks).
Figure 1
Borders of the Wielkopolska National Park and location of the study area in Poland
Based on OpenStreetMap: https://www.openstreetmap.org/
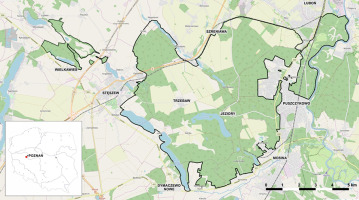
Figure 2
Jarosławieckie Lake – a ribbon lake in between the villages of Jeziory and Trzebaw in the WPN. Photography by Anna Kujawa.

The first data on fungi from the surroundings of Poznań was provided by Pfuhl (Pfuhl,1896,1898) at the end of the nineteenth century. At least some of them concerned the area currently included in the WPN (e.g., forests in the vicinity Mosina, Wiry, or Szreniawa). Regular mycological studies in the WPN began in 1928 by N. Szyndlerówna and have been continued to the present day. Part of these results have been published in numerous monographs (Bałazy,1988; Domański,1965,1991; Domański et al.,1967; Lisiewska,1961; Nespiak,1975,1981,1990; Skirgiełło,1998,2000), articles (Bałazy,1982; Bujakiewicz,1973; Bujakiewicz & Fiebich,1992,1993; Danielewicz & Maliński,1999; Domański,1955,1960; Dzięczkowski,1961; Fiedotjew,1936; Jesse,1947; Kujawa, Gierczyk, et al.,2012; Lisiewska,1965,2011; Lisiewska & Madeja,2003; Młynarek,1971; Pfuhl,1896,1898; Raszka,1994; Skirgiełło,1976,1984; Skirgiełło & Wosińska,1963; Szulczewski,1931; Teodorowicz,1932,1933; Wojewoda,1977,1979), and conference proceedings (Bujakiewicz,1992), while others remain unpublished in master’s theses (Fiebich,1989; Pawlak,1977; Skowrońska,1986; Susicka,1994; Szyndlerówna,1928; Wilczyńska,1994) and expertises (Bujakiewicz & Fiebich,1991; Bujakiewicz & Szambelańczyk,1997). The total number of taxa mentioned from the WPN in 2017 in published and unpublished materials is 814, including species of uncertain status (Kujawa,2017; Lisiewska,2011). Among them, 622 were listed in published reports. In 2018, a new project on the fungal diversity in the WPN started. In the beginning, the number of taxa known from the WPN and mentioned in all available sources was verified; the hitherto known mycobiota of this Park composed of 786 taxa. Moreover, 50 species of uncertain status have been reported in published and unpublished materials. In this paper, an annotated list of localities of 140 taxa, new for the WPN, and found during the first year of the study is presented. The list of taxa previously reported from the WPN and confirmed in 2018 is also enclosed.
Material and Methods
Specimens were collected in 2018 from March to November, using the route method. All types of habitats in the WPN were checked during the field studies. Forest compartment numbers were given after the Forest Data Bank (https://www.bdl.lasy.gov.pl/). The specimens collected were identified using standard methods used in fungal taxonomy, i.e., analysis of micro- and macrocharacters using a binocular or optical microscope. Standard staining techniques using aqueous ammonia solution, 10% KOH in water, Congo red in ammonia, Melzer reagent, sulfovanilin, and aniline (cotton) blue in lactophenol were applied. Specimens were identified using the following general keys and atlases: Funga Nordica (Knudsen & Vesterholt,2008,2012), Nordic Macromycetes (Hansen & Knudsen,1992,1997,2000), Flora Agaricina Neerlandica (Bas et al.,1988,1990,1995,1999; Noordeloos et al.,2001,2005), Die Nichtblätterpilze, Gallertpilze und Bauchpilze (Jülich,1984), Fungi of Switzerland (Breitenbach & Kränzlin,1984,1986,1991,1995), and Pilzkompendium (Ludwig,2000,2001,2007,2007,2012,2012,2017,2017). The following monographs and taxonomic papers were used: Agaricus (Parra,2008,2013), Conocybe and Pholiotina (Hausknecht,2009), Crepidotus (Consiglio & Setti,2008), Hebeloma (Beker et al.,2016), Hypocrea (Jaklitsch,2009,2011), Peziza (Hohmeyer,1986), Scutellinia (Schumacher,1990), Boletales (Ladurner & Simonini,2003; Lannoy & Estades,1995), Strophariaceae (Noordeloos,2011), polyporoid fungi (Bernicchia,2005; Ryvarden et al.,2017), corticioid fungi (Bernicchia & Gorjón,2010). Specimens of Ascomycota were also identified based on the keys, iconography, and descriptions provided in a DVD edition by Baral & Maron (2005). For the taxa mentioned in Funga Nordica 2 (Knudsen & Vesterholt,2012), the names given in this monograph have been used (except for a few taxa, as indicated on the species list), other names have been used as recommended in MycoBank Database (http://www.mycobank.org/). The data on the distribution in Poland were compiled based on the checklists of Polish micro- and macromycetes (Chmiel,2006; Mułenko et al.,2008; Wojewoda,2003) and the database of Polish mycological literature (Kujawa,2018). The threat categories were given based on the “Red list of the macrofungi in Poland” (Wojewoda & Ławrynowicz,2006), the protected species – according to the Regulation of the Minister of Environment (2014). Dry specimens were deposited in B. Gierczyk private fungarium and Turew Field Station of the Institute for Agricultural and Forest Environment of the Polish Academy of Sciences.
Results
Abbreviations:
! – species new to Poland, # – species new to Wielkopolska region;
LP – Landscape Park, NP – National Park, NR – Nature Reserve;
PU – Protection Unit (Polish: Obwód Ochronny): Jz – Jeziory; Gr – Górki; OG – Osowa Góra; Pu – Puszczykowo; Wi – Wiry; Wy – Wypalanki;
NICL – species not included in Polish checklists; RL – species mentioned in red list with categories: Ex – extinct, E – endangered, V – vulnerable, R – rare;
Ac.ng – Acer negundo; Ac.pl – Acer platanoides; Ac.ps – A. pseudoplatanus; Al.g – Alnus glutinosa; Bt – Betula spp.; Bt.p – B. pendula; Co – Corylus avellana; Cp – Carpinus betulus; Fg – Fagus sylvatica; Fx – Fraxinus excelsior; La – Larix sp.; Pi – Pinus sylvestris; Po – Populus spp.; Q – Quercus spp.; Rb – Robinia pseudoacacia; Sa – Salix spp.; Ti – Tilia spp.; Um – Ulmus spp.
Annotated Species List: Ascomycota
Bisporella citrina (Batsch) Korf & S. E. Carp.
Specimens Examined
1. Puszczykowo, 1.5 km E (PuPU: 144); X/2018; Pi forest; numerous apothecia on twigs of a deciduous tree. 2. Puszczykowo, 2.5 km NWW (JzPU: 74); X/2018; oak-hornbeam forest (Q); numerous apothecia on wood of Q.
Ciliolarina laricina (Raitv.) Svrček
Dialonectria episphaeria (Tode) Cooke
Specimens Examined
1. Ludwikowo, 700 m N (OGPU: 90; by Kociołek Lake); IX/2018; mixed forest (Pi, Q, Fg, Bt, Ac.ps); numerous perithecia on Hypoxylon sp. stromata on Fg log. 2. Puszczykowo, 1.5 km E (PuPU: 14); X/2018; Pi forest; numerous perithecia on Hypoxylon sp. stromata on Fg twigs. 3. Stare Puszczykowo (PuPU: 54); XI/2018; oak-hornbeam forest; numerous perithecia on Hypoxylon fragiforme stromata on twigs of a deciduous tree.
Hypocrea strictipilosa P. Chaverri & Samuels; NICL
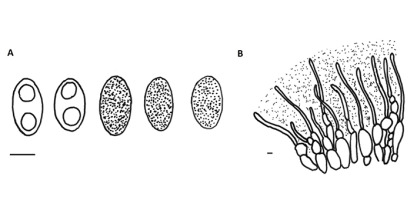
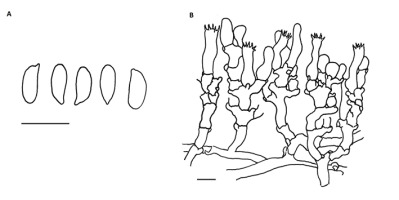
!Pachyella violaceonigra (Rehm) Pfister; NICL (Figure 3)
Specimen Examined
Jarosławiec, 1.5 km NW (WiPU: 103); IX/2018; Al.g forest; a few ascomata on Al.g log.
Notes
Apothecia up to 3.5 cm in diameter, shallowly cup-shaped when young, then applanate to slightly pulvinate, discoid, appressed to substratum with somewhat undulate margin, fleshy and fragile. Hymenial layer dark to very dark chestnut brown with distinct vinaceous red or violaceous tint. The outer surface of the ascomata paler. Asci eight-spored, amyloid in the upper part, with crosiers. Spores ellipsoid, thin-walled, hyaline with two guttules, 20–30 × 12–14 µm, mature spores covered with fine warts and low and short coma-like ridges. Paraphyses cylindrical with inflated apex, septate, with dark bodies in upper part. Ectal excipulum of textura globulosa with hyphoid, gel-embedded outer layer. Medullary excipulum of textura intricata.
#Peziza pseudovesiculosa Donadini; NICL
Scutellinia patagonica (Rehm) Gamundí; NICL
Basidiomycota
#Agaricus cupreobrunneus (F. H. Møller) Pilát
Agaricus impudicus (Rea) Pilát; NICL
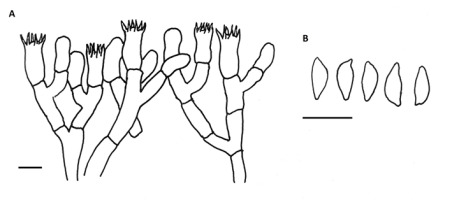
Agaricus porphyrrhizon P. D. Orton (Figure 4 )
Agrocybe putaminum (Maire) Singer; NICL
#Amylocorticium subincarnatum (Peck) Pouzar; RL-V
Armillaria borealis Marxm. & Korhonen
Specimens Examined
1. Puszczykowo, 2.5 km NWW (JzPU: 74); X/2018; mixed forest (Pi, Q, Cp, Ti, Co); numerous basidiomata on soil. 2. Wielkawieś, 0.6 km N (WyPU: 122K); X/2018; riparian forest, numerous basidiomata on soil. 3. Between Wypalanki and DK5 road (WyPU); XI/2018; deciduous and mixed forests; very numerous basidiomata on soil, trunks, and logs. 4. Łęczyca, 2.2 km NEE (PuPU: 4); XI/2018; mixed forest; numerous basidiomata on stumps and dying oaks. 5. Wielkawieś, 0.8 km NNW (WyPU: 123K); X/2018; riparian forest; numerous basidiomata on stumps and logs.
Armillaria mellea (Vahl) P. Kumm. s. str. (Figure 5)
Specimens Examined
1. Puszczykowo, 3 km W (JzPU: 75); X/2018; mixed forest (Pi, Q, Cp, Ti, Co); numerous basidiomata on the base of a trunk of a deciduous tree. 2. Wielkawieś, 1.2 km NNW (WyPU: 124K); X/2018; riparian forest; numerous basidiomata on the base of a trunk of a deciduous tree. 3. Wielkawieś, 0.8 km NNW (WyPU: 123K); X/2018; riparian forest; numerous basidiomata on trunks, logs, and basis of dying trees.
Armillaria ostoyae (Romagn.) Herink
Specimens Examined
1. Łęczyca, 2.2 km NEE (PuPU: 4); XI/2018; mixed forest; numerous basidiomata on soil, trunks, and dying trees. 2. Wielkawieś, 0.6 km N (WyPU: 122K); X/2018; riparian forest; numerous basidiomata on soil. 3. Between Wypalanki and DK5 road (WyPU); XI/2018; deciduous and mixed forests; very numerous on soil, trunks, and logs.
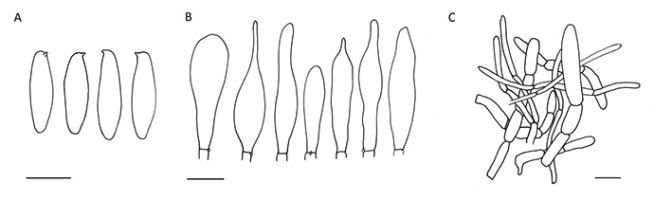
!Botryobasidium robustius Pouzar & Hol.-Jech.; NICL (Figure 6)
Specimen Examined
Wypalanki, 2 km S (WyPU: 179); XI/2018; mixed forest (Q, Pi, Ac.ps, Fg, Fx, Co); a few basidiomata on wood of a deciduous tree.
#Coniophora olivacea (Pers.) P. Karst.; RL-R
Specimens Examined
1. Mosina, 3 km NWW (OGPU: 136); X/2018; mixed forest (Q, Pi, Cp); a few basidiomata on Pi log. 2. Jarosławiec, 0.7 km SE (JzPU: 114); X/2018; mixed forest (Q, Pi, Cp); a few basidiomata on Pi log. 3. Puszczykowo, 3 km W (JzPU: 75); X/2018; mixed forest (Pi, Q, Cp, Co, Ti); a few basidiomata on Pi log.
#Crepidotus malachius Peck. var. trichifer Hesler & A. H. Sm.; NICL
Specimens Examined
1. Puszczykowo, 3 km W (JzPU: 75); X/2018; mixed forest (Pi, Q, Cp, Ti, Co); numerous basidiomata on a log of a deciduous tree. 2. Puszczykowo, 1.5 km E (PuPU: 14); X/2018; Pi forest; one basidioma on Pi trunk. 3. Puszczykowo, 1.7 km N (PuPU: 18); X/2018; floodplain terrace (Sa, Ac.ng, Po); numerous basidiomata on Pi log.
#Dendrothele dryina (Pers.) P. A. Lemke; RL-Ex
!Hebeloma subtortum P. Karst.; NICL (Figure 7)
Specimen Examined
Rosnówko, 2 km SEE (WiPU: 116A); X/2018; young deciduous forest; numerous basidiomata on soil.
Notes
Representative of section Hebeloma (Fr.) P. Kumm. Cap hemispherical to broadly campanulate, then almost applanate to indistinctly concave, 4–7 cm in diameter, fleshy and firm, smooth, not hygrophanous, cream-colored to pale ochre or pale buff. Stipe cylindrical, fibrillose, 4–7 × 0.6–1.4 cm, almost white. Lamellae 55–70, adnate to emarginate, with fimbriate margin, without tears. Cortina present, white. Smell and taste raphanoid. Basidia four-spored. Spores ovoid to ovoid-ellipsoid, 6.5–11.5 × 4.5–7 µm, brownish yellow, weakly ornamented, indextrinoid, perispore not loosening. Cheilocystidia lageniform to subcylindrical, ventricose, not constricted, ca. 40–60 µm long, with 4.5–5.5 µm wide apex and 7–12 µm broad basal part. Pleurocystidia absent. Pileipellis an ixocutis ca. 250 µm thick, composed of encrusted hyphae. Caulocystidia similar to cheilocystidia.
Helicogloea lagerheimii Pat.; NICL
Hyphoderma medioburiense (Burt) Donk; NICL
Hyphoderma nemorale K. H. Larss.; NICL
Hyphoderma setigerum (Fr.) Donk
Specimens Examined
1. Górka, 1.4 km E (GrPU: 126); X/2018; mixed forest (Q, Pi, Cp); a few basidiomata on Cp log. 2. Jarosławiec, 1.5 km NW (WiPU: 103); X/2018; mixed forest (Q, Pi, Cp); a few basidiomata on Padus sp. log. 3. Wielkawieś, 0.8 km NNW (WyPU: 123K); X/2018; riparian forest; a few basidiomata on a log of a deciduous tree.
Hypochnicium wakefieldiae (Bres.) J. Erikss.
Ischnoderma benzoinum (Wahlenb.) P. Karst.; RL-V
Specimens Examined
1. Puszczykowo, 2.5–3 km W (JzPU: 74, 75); X/2018; mixed forest (Pi, Q, Cp, Ti, Co); a few basidiomata on Pi log. 2. Wypalanki, 2 km S (WyPU: 179); XI/2018; mixed forest (Q, Pi, Ac.ps, Fg, Fx, Co); a few basidiomata on Pi log. 3. Puszczykowo, 1.5 km E (PuPU: 14); X/2018; Pi forest; a few basidiomata on Pi trunk.
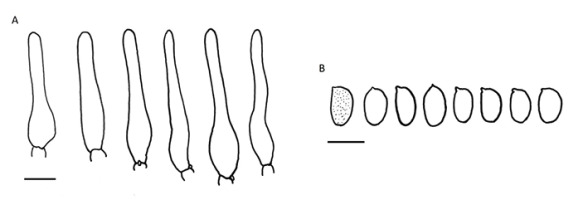
!Leccinum brunneogriseolum Lannoy & Estadès; NICL (Figure 8)
Specimen Examined
Jarosławiec, 1.5 km NW (WiPU: 103); IX/2018; Al.g forest, under Bt, Al.g, Sa; a few basidiomata on soil.
Notes
Cap nut brown to ochre brown, hemispherical, minutely granulose or tomentose, 3–5 cm in diameter. Stipe indistinctly clavate, ca. 10 cm high, whitish, covered with brownish squamules. Pore surface white when young, becoming pale grayish-cream colored with age. Context almost white, becoming greenish-blue in the lower part of stipe and pale pink in stipe apex. Spores boletoid to narrowly fusiform with subhilar depression, brownish yellow, 12–23 × 4–7 µm, Qav > 3. Basidia four-spored. Hymenial cystidia fusiform, lageniform to subcylindrical. Pileipellis a trichoderm, composed of filamentous, twisted hyphae and chain-forming, easily detachable cylindrocystis 3.5–7 µm wide; terminal elements cylindrical to conical, pigment intracellular, brown. Caulocystidia in clusters, intermixed with scattered basidia, variable – cylindrical, utriform, fusiform, narrowly clavate, or lageniform, often with a flexuous neck. Species variously interpreted, sometimes considered as a synonym of L. cyaneobasileucum Lannoy & Estadès (Knudsen & Vesterholt,2012; Mikšík,2017), its variety [L. cyaneobasileucum Lannoy & Estadès var. brunneogriseolum (Lannoy & Estadès) Lannoy & Estadès] (Noordeloos et al.,2018) or a separate species (Lannoy & Estades,1995).
Leucogyrophana mollusca (Fr.) Pouzar
Specimens Examined
1. Mosina, 3 km NWW (OGPU: 136); X/2018; mixed forest (Q, Pi, Cp); a few basidiomata on Pi log. 2. Puszczykowo, 2.5 km NWW (JzPU: 74); X/2018; mixed forest (Pi, Q, Cp, Ti, Co); a few basidiomata on Pi log. 3. Wypalanki, 2.5 km S (WyPU: 183); XI/2018; mixed forest (Q, Pi, Cp, Co); a few basidiomata on Pi log.
#Leucogyrophana romelii Ginns; NICL
Lyomyces juniperi (Bourdot & Galzin) Riebesehl & E. Langer; NICL
Macrolepiota konradii (Huijsman ex P. D. Orton) M. M. Moser
Specimens Examined
1. Puszczykowo, 2.5–3 km W (JzPU: 74, 75); X/2018; mixed forest (Pi, Q, Cp, Ti, Co); a few basidiomata on soil. 2. Wielkawieś, 1.2 km NNW (WyPU: 124K); X/2018; mixed forest (Pi, Q, Bt, Cp, Um); a few basidiomata on soil. 3. Łęczyca, 2.2 km NEE (PuPU: 4); XI/2018; Pi forest; one basidioma on soil.
Meruliopsistaxicola (Pers.) Bondartsev; RL-R (Figure 9)
#Mucronella flava Corner (Figure 10)
Naucoria badiolateritia P. D. Orton; NICL
Specimen Examined
Jarosławiec, 1.5 km NW (WiPU: 103); X/2018; alder forest by the drying lake; very numerous basidiomata on soil.
Notes
In Poland also known from the vicinity of Nowy Dworek near Świebodzin (Gierczyk & Ślusarczyk,2020).
Peniophora quercina (Pers.) Cooke
Specimens Examined
1. Wielkawieś, 1.2 km NNW (WyPU: 124K); X/2018; mixed forest (Pi, Q, Bt, Cp, Um); a few basidiomata on Q branch. 2. Łęczyca, 2.2 km NEE (PuPU: 4); XI/2018; riparian forest; a few basidiomata on Q branch. 3. Puszczykowo, 1.5 km SEE (PuPU: 19); X/2018; Q forest; numerous basidiomata on Q branches. 4. Jarosławiec, 1 km N (98, 99); IX/2018; mixed forest; a few basidiomata on Q branch.
Peniophorella pubera (Fr.) P. Karst.
Specimens Examined
1. Wypalanki, 2.2 km SSW (WyPU: 184); XI/2018; deciduous forest (Q, Fg, Cp, Fx, Ac.pl); a few basidiomata on a log of a deciduous tree. 2. Mosina, 3 km NWW (OGPU: 136); X/2018; mixed forest (Q, Pi, Cp); a few basidiomata on Q log. 3. Mosina, 2.5 NWW (OGPU: 90; by Lake Kociołek); IX/2018; mixed forest (Fg, Q, Pi, Bt, Ac.ps); a few basidiomata on a log of a deciduous tree.
Phanerochaete laevis (Fr.) J. Erikss. & Ryvarden
#Phlebia subochracea (Bres.) J. Erikss. & Ryvarden, RL-Ex (Figure 11)
#Pluteus cervinus (Schaeff.) P. Kumm. f. albus Peck
Polyporus badius (Pers.) Schwein.
Specimens Examined
1. Jarosławiec, 1 km SE (JzPU: 115); VIII/2018; mixed forest; a few basidiomata on a log of a deciduous tree. 2. Jarosławiec, 1.5 km NW (WiPU: 103); X/2018; alder forest by the drying lake; a few basidiomata on wood. 3. Wielkawieś, 0.6–0.8 km N (WyPU: 122K, 123K); X/2018; riparian forest, few basidiomata on a log of a deciduous tree.
Postia tephroleuca (Fr.) Jülich
Specimens Examined
1. Puszczykowo, 1.7 km N (PuPU: 18); X/2018; mixed forest (Q, Pi, Cp, Ac.pl, Ac.ps); a few basidiomata on a log of a deciduous tree. 2. Wielkawieś, 0.6 km N (WyPU: 122K); X/2018; mixed forest (Q, Pi, Um, Cp, Bt), few basidiomata on a log of a deciduous tree. 3. Trzebaw, 1.3 km SSW (GrPU: 204); XI/2018; Pi forest; a few basidiomata on Bt branch.
#Psathyrella fibrillosa (Pers.) Maire
#Psathyrella pannucioides (J. E. Lange) M. M. Moser
#Resinicium furfuraceum (Bres.) Parmasto; NICL
#Rigidoporus undatus (Pers.) Donk
!Sistotrema athelioides Hallenb. (Figure 12)
Specimen Examined
Rosnówko, 0.4 km W (WyPU: 177); XI/2018; mixed forest (Pi, La, Fg, Q); a few basidiomata on a branch of a deciduous tree.
#Trechispora subsphaerospora (Litsch.) Liberta; NICL
Xerocomus ripariellus Redeuilh; NICL
Xylodon nesporii (Bres.) Hjortstam & Ryvarden
Specimens Examined
1. Jarosławiec, 0.7 km SE (JzPU: 114); VIII/2018; mixed forest (Pi, Q, Cp); a few basidiomata on Pi log. 2. Wielkawieś, 0.6 km N (WyPU: 122K); X/2018; riparian forest; a few basidiomata on a log of a deciduous tree. 3. Wypalanki, 2.5 km S (WyPU: 183); XI/2018; mixed forest (Q, Pi, Cp, Co); a few basidiomata on Pi log.
List of Taxa Reported from WPN Previously and Confirmed in 2018
Ascomycota
Adelphella babingtonii (Berk. & Broome) Pfister, Matočec & I. Kušan (103)3; Ascocoryne cylichnium (Tul.) Korf (49); Bulgaria inquinans (Pers.) Fr. (49, 177); Encoelia furfuracea (Roth) P. Karst. (74); Hymenoscyphus scutula (Pers.) W. Phillips (67); Hypoxylon fuscum (Pers.) Fr. (74); Nectria cinnabarina (Tode) Fr. (54); Peziza micropus Pers. (4); Pezizella alniella (Nyl.) Dennis; (122); Rhytisma acerinum (Pers.) Fr. (4); Xylaria hypoxylon (L.) Grev. (16, 49, 75, 123, 179, 183, 184, by Dymaczewskie Lake).
Basidiomycota
Amanita citrina (Schaeff.) Pers. f. citrina (14); A. muscaria (L.) Hook. var. muscaria (204); Auricularia auricula-judae (Bull.) J. Schröt. (14, 122, 136, 177); Auriscalpium vulgare Gray (54, 123); Baeospora myosura (Fr.) Singer (4, 14, 18, 49, 69, 74, 123, 184, 204); Bjerkandera adusta (Willd.) P. Karst. (4, 74, 90); Bolbitius reticulatus (Pers.) Ricken f. aleuriatus (Fr.) Enderle (49); Botryohypochnus isabellinus (Fr.) J. Erikss. (74); Bovista nigrescens Pers. (123); Bulbillomyces farinosus (Bres.) Jülich (14); Calocera cornea (Batsch) Fr. (75); Ceraceomyces serpens (Tode) Ginns (119, 179); Chondrostereum purpureum (Pers.) Pouzar (4, 14, 123); Clitocybe nebularis (Batsch) P. Kumm. var. nebularis (54, 74, 182); C. odora (Bull.) P. Kumm. var. odora (124); Clitocybula platyphylla (Pers.) Malençon & Bertault (90); Clitopilus hobsonii (Berk.) P. D. Orton (49, 177, 183); Conocybe echinata (Velen.) Singer (166); Coprinellus disseminatus (Pers.) J. E. Lange (14, 184); C. domesticus (Bolton) Vilgalys, Hopple & Jacq. Johnson (116); C. micaceus (Bull.) Vilgalys, Hopple & Jacq. Johnson (49, 171); C. xanthothrix (Romagn.) Vilgalys, Hopple & Jacq. Johnson (14, 54, 124, 127, 169); Coprinopsis atramentaria (Bull.) Redhead, Vilgalys & Moncalvo (74); Coprinus comatus (O. F. Müll.) Pers. (69); Crepidotus variabilis (Pers.) P. Kumm. (4, 54, 75); Dacrymyces stillatus Nees (174); Daedalea quercina (L.) Pers. (35, 124); Daedaleopsis confragosa (Bolton) J. Schröt. (4, 14, 103, 108, 122, 204, between Górka and Trzebaw); Datronia mollis (Sommerf.) Donk (49, 122); Echinoderma aspera (Pers.) Bon (136); Entoloma clypeatum (L.) P. Kumm. var. clypeatum (208); Exidia nigricans (With.) P. Roberts (4, 90, 123, 124, 177, 179); E. saccharina (Alb. & Schw.) Fr. (74); E. truncata Fr. (74, 90, 176, 177); Flammulina velutipes (Curtis) P. Karst. var. velutipes (14, 49, 122, 123); Fomes fomentarius (L.) Fr. (4, 18, 49, 90, 103, 110, 122, 124, 179, 184, 211, 218, 219, 220, 221, near forest compartment No. 25, by Dymaczewskie Lake); Fomitopsis pinicola (Sw.) P. Karst. (83, 124); Galerina hypnorum (Schrank) Kühner s. Horak 2005 and de Haan & Walleyn 2006 (18); G. marginata (Batsch) Kühner s.l. (75, 122, 176); G. triscopa (Fr.) Kühner (74); Ganoderma australe (Fr.) Pat. (219); G. lipsiense (Batsch) G. F. Atk. (14, 18, 49, 74, 75, 90, 103, 119, 122, 124, 183, 219, by Dymaczewskie Lake); Geastrum triplex Jungh. s. auct. (91, by Dymaczewskie Lake); Gloeophyllum trabeum (Pers.) Murrill (85); Gymnopilus penetrans (Fr.) Murrill (4, 14, 18, 74, 179); G. picreus (Pers.) P. Karst. (75); G. spectabilis (Weinm.) A. H. Sm. (14); Hapalopilus nidulans (Fr.) P. Karst. (74, 83, 90); Hebeloma mesophaeum (Pers.) Quél. (166); Hemipholiota populnea (Pers.) Bon (28); Heterobasidion annosum (Fr.) Bref. s. l. (14, 74, 183, by Dymaczewskie Lake); Hygrophoropsis aurantiaca (Wulfen) Maire (4, 14, 18, 19, 74, 103, 169, 174, 175, 176, 184, 204, 208); Hymenochaete rubiginosa (Schrad.) Lév. (54, 65, 83, 113, 119, 122, 177); Hyphoderma praetermissum (P. Karst.) J. Erikss. & Å. Strid (183); Hyphodontia sambuci (Pers.) J. Erikss. (74, 110); Hypholoma capnoides (Fr.) P. Kumm. (14, 124); H. fasciculare (Huds.) Kumm. var. fasciculare (4, 14, 19, 47, 49, 74, 91, 122, 136, 170, 177, 178, 181, 182, 183, 184, 204, 205, 208); H. lateritium (Schaeff.) P. Kumm. (14, 124); Infundibulicybe gibba (Pers.) Harmaja (67); Inonotus radiatus (Sowerby) P. Karst. (74, 75, 103, 177); Kuehneromyces mutabilis (Schaeff.) Singer & A. H. Sm. (74); Lactarius quietus (Fr.) Fr. (19); Laetiporus sulphureus (Bull.) Murrill (83, 90, 103, 210, 222, between Górka and Trzebaw); Lepiota cristata (Bolton) P. Kumm. (29, 126); Lepista nuda (Bull.) Cooke (123); L. sordida (Schumach.) Singer (166); Lycoperdon pyriforme Schaeff. (49, 75, 90, 119, 123); Macrolepiota excoriata (Schaeff.) Wasser (222); M. procera (Scop.) Singer (14, 15, 115, 124, 127, 204, 210, between Górka and Trzebaw); Macrotyphula filiformis (Bull.) Rauschert (74); Marasmius setosus (Sowerby) Noordel. (18, 54); Melanoleuca subpulverulenta (Pers.) Métrod (124); Mycena acicula (Schaeff.) P. Kumm. (136); M. capillaris Peck. (54); M. filopes (Bull.) P. Kumm.; (4, 74, 204); M. galericulata (Scop.) Gray (14, 18, 19, 49, 74, 75, 90, 103, 113, 119, 122, 123, 126, 127, 170, 176, 177, 178, 179, 181, 182, 183, 184); M. galopus (Pers.) P. Kumm. (4, 67, 183, 208); M. haematopus (Pers.) P. Kumm. (18, 90, 123); M. leptocephala (Pers.) Gillet (74); M. polyadelpha (Lasch) Kühner (74); M. polygramma (Bull.) Gray (4, 54, 75, 124); M. pseudocorticola Kühner (49); M. pura (Pers.) P. Kumm. (123); M. speirea (Fr.) Gillet (183); M. stipata Maas Geest. & Schwobel (14, 74, 75, 126); M. tintinabulum (Fr.) Quél. (177); M. vitilis (Fr.) Quél. (49, 90, 123, 124, 179, 181); M. zephirus (Fr.) P. Kumm. (4, 14, 18, 49, 67,169, 171, 175, 208); Mycetinis querceus (Britzelm.) Antonín & Noordel. (176); Naucoria scolecina (Fr.) Quél. (177); N. subconspersa Kühner (103); N. submelinoides (Kühner) Maire (14); Neolentinus lepideus (Fr.) Redhead & Ginns (90, 83, 108, 119); Panaeolus cinctulus (Bolton) Sacc. (166); Panellus stipticus (Bull.) P. Karst. (90); Parasola conopilus (Fr.) Örstadius & E. Larss. (123); P. plicatilis (Curtis) Redhead, Vilgalys & Hopple (127); Paxillus involutus (Batsch) Fr. s. l.; (4, 19, 204); P. rubicundulus P. D. Orton s. l. (166); Peniophora cinerea (Pers.) Cooke (114, 124); Phaeolus schweinitzii (Fr.) Pat. (18, 74); Phanerochaete tuberculata (P. Karst.) Parmasto (124); Phellinus ferruginosus (Schrad.) Pat. (85); P. igniarius (L.) Quél. s. l. (14, 103, by Dymaczewskie Lake, near Łódź forester lodge); P. robustus (P. Karst.) Bourdot & Galzin (36, 37, 74, 91, 98/104); P. tuberculosus (Baumg.) Niemelä (91); Phlebia radiata Fr. (19, 49, 74, 75, 122, 123, 126, 184); P. rufa (Pers.) M. P. Christ. (126); P. tremellosa (Schrad.) Nakasone & Burds. (14, 18, 49, 75, 90, 177); Pholiota lucifera (Lasch) Quél. (123); P. squarrosa (Weigel) P. Kumm. (75, 122, 123); Piptoporus betulinus (Bull.) P. Karst. (87, 123, 124, 204, 208); Pleurotus ostreatus (Jacq.) P. Kumm. (4, 28, 49); Plicatura crispa (Pers.) Rea (183); Pluteus cervinus (Schaeff.) P. Kumm. f. cervinus (4, 14, 49, 74, 90, 99, 124, 175, 183, 184, 208, 211, by Dymaczewskie Lake); P. podospileus f. minutissimus (Maire) Vellinga (103); P. salicinus (Pers.) P. Kumm. (14); Polyporus brumalis (Pers.) Fr. (75); P. squamosus (Huds.) Fr. (90, 91, 160, 203, 218, 219, 220, 221); Porodaedalea pini (Brot.) Murrill (4, 14, 18, 19, 29, 49, 74, 91, 98, 99, 100, 101, 123, 136, 177); Psathyrella candolleana (Fr.) Maire (74); P. corrugis (Pers.) Konrad & Maubl. (124, 127, 169); P. fatua (Fr.) Konrad & Maubl. (126, 127); P. microrrhiza (Lasch) P. Kumm. (69, 170); P. noli-tangere (Fr.) A. Pearson & Dennis (103, 179); P. piluliformis (Bull.) P. D. Orton (174, 183); P. spadicea (P. Kumm.) Singer (47, 204); Radulomyces confluens (Chaillet) M. P. Christ. (177); Ramaria stricta (Pers.) Quél. (49); Rhodocollybia butyracea (Bull.) Lennox f. asema (Fr.) Antonín, Halling & Noordel (4, 49, 74, 170, 179); Rickenella fibula (Bull.) Raithelh. (14, 18, 75, 179); R. swartzii (Fr.) Kuyper (67); Russula cyanoxantha (Schaeff.) Fr. (166); R. ochroleuca (Pers.) Fr. (54, 123); Sarcomyxa serotina (Pers.) P. Karst. (74, 75, 123, 183); Schizophyllum commune Fr. (90, 174); Scleroderma citrinum Pers. (90); Serpula himantioides (Fr.) P. Karst. (74, 136, 182, 183); Sidera vulgaris (Fr.) Miettinen (169); Simocybe haustellaris (Fr.) Watling (49); Sparassis crispa (Wulfen) Fr (46, 81); Steccherinum ochraceum (Pers. ex J. F. Gmel.) Gray (124); Stereum hirsutum (Willd.) Pers. (4, 14, 74, 123); S. rugosum Pers. (49, 91); S. subtomentosum Pouzar (90, 177); Strobilurus tenacellus (Pers.) Singer (36, 65, 113, 119, by Dymaczewskie Lake); Stropharia coronilla (Bull.) Quél. (126); S. cyanea (Bull.) Tuom. (68, 85); Suillus granulatus (L.) Roussel (75); Tapinella atrotomentosa (Batsch) Šutara (115); Trametes hirsuta (Wulfen) Pilát (14, 90, 122); T. ochracea (Pers.) Gilb. & Ryvarden (184); T. versicolor (L.) Pilát (4, 18, 90, 122, 123, 177, 183, 184); Tremella mesenterica Retz. (18, 49, 177); Trichaptum abietinum (Pers. ex J. F. Gmel.) Ryvarden (74, 110); T. hollii (J. C. Schmidt) Kreisel (4, 91, 183, 208); Tricholoma terreum (Schaeff.) P. Kumm. s. l. (75); Tubaria furfuracea (Pers.) Gillet s. l. (123, 127); Typhula erythropus (Pers.) Fr. (122); Xylodon paradoxus (Schrad.) Chevall. s. l. (74, 87, 114, 136, 179).
Discussion
Dry and warm summer in 2018 drastically limited the richness of fungi observed in the WPN. Remarkably poor fructification was noted for agaricoid fungi, e.g., no Inocybe or Cortinarius specimens were collected. In total, 312 taxa of macrofungi were observed in the WPN in 2018. Among them, 140 have not been previously reported from the WPN. Five species have been collected for the first time in Poland (Botryobasidium robustius, Hebeloma subtortum, Leccinum brunneogriseolum, Pachyella violaceonigra, and Sistotrema athelioides). Another 26 taxa were new for the Wielkopolska region. Some taxa observed in the WPN are very rare and have been found in the recent years for the first time in Poland (e.g., Crepidotus malachius var. trichifer, Leucogyrophana romelii, and Resinicium furfuraceum) or were even recorded as new to Poland in 2018 from the Wielkopolska region, independently of the project conducted in the WPN (e.g., Helicogloea lagerheimii). Two taxa regarded as extinct in Poland (Dendrothele dryina, and Phlebia subochracea) were also collected in the Park. The current number of taxa known from the WPN is 975 (including those of uncertain status). The presented results and analysis of available data suggest that further studies should be focused on wood inhabiting fungi, especially the polyporoid and corticioid taxa, as well as on ascomycetes because the richness and diversity of these groups in the WPN are underestimated and are poorly known. The continuation of the studies in the WPN would also permit confirmation or verification of the occurrence of the taxa reported from this area in the first half of the twentieth century, which has not been observed again since then, e.g., Auricularia mesenterica, Geastrum lageniforme, Gyromitra leucoxantha (Jesse,1947), Clavulina amethystina or Discina ancilis (Jesse,1947; Teodorowicz,1933); some historical data had already been confirmed in 2018 (e.g., the occurrence of Botryohypochnus isabellinus, Macrolepiota excoriata, and Panaeolus cinctulus). Depending on the weather conditions in the coming years, the results of the planned field research may be miscellaneous, as the fungi growth and sporocarps development depend strongly on weather conditions. Regardless of the method of study, i.e., permanent plot, transect, or random route methods, the biology of fungi implies that the research duration should be of at least 5–6 years (Friedrich,2008; Szczepkowski & Kujawa,2018). Moreover, the extent of the area and high habitat diversity in the WPN suggest that even a few-year-long inventory will provide incomplete data on the fungal richness, chorology, and diversity. Therefore, independent, multifaceted mycological scientific projects should be initiated in the WPN.
Handling Editor
Andrzej Szczepkowski; Warsaw University of Life Sciences – SGGW, Poland; https://orcid.org/0000-0002-9778-9567