Introduction
Epicoccum nigrum Link (syn. E. purpurascens Ehrenb. ex Schlecht) is an endophytic fungal species, which is widely distributed as it is found on plant surfaces and in water, soil, and air. This species is particularly known for producing a variety of biologically-active substances. Epicoccum nigrum isolated from the marine environment produces extracellular polysaccharides with free radical scavenging activity and is potentially useful in the prevention of oxidative damage in higher organisms (Sun et al.,2011). Somjaipeng et al. (2016) showed that E. nigrum could also produce taxol, which is a diterpenoid anticancer drug, and is induced by the elicitors, like water activity or pH. Colored secondary metabolites, such as prodigiosins, which are also excreted by E. nigrum, may have a potential role as antimicrobial or antitumor compounds (Perveen et al.,2017). Epicoccum nigrum extract, which was isolated from the Ferula sumbul leaves, was also found to contain prodiginine and was shown to exhibit strong antimicrobial activity against the microscopic fungi and bacteria (e.g., Bacillus subtilis, Escherichia coli, Staphylococcus aureus, and Candida albicans). It was also shown to exhibit anticancer activities against melanoma cell lines (Perveen et al.,2017). This fungus, when isolated from the cambium of Phellodendron amurense, has also been used for the extracellular synthesis of silver nanoparticles with a wide variety of biological activities (Qian et al.,2013). Epicorazines A and B, isolated from E. nigrum, exhibited antibacterial activity. Cultured E. nigrum hyphae were also shown to excrete several dyes, including β- and γ-carotene, rhodoxantin, and epicocconone, in the medium (Baute et al.,1978).
The variety of biologically-active secondary metabolites produced by E. nigrum makes it a potential organism for the biocontrol of phytopathogens. Although there is one report describing the pathogenic interaction of E. nigrum with Lotus corniculatus (Colavolpe et al.,2018), in general, this fungus is considered to be a facultative saprotroph, exhibiting an important role in plant protection against pathogens (de Cal et al.,2009). It has previously been shown that E. nigrum isolated from sugarcane inhibits several phytopathogens, such as Cyanophora paradoxa and Fusarium verticilloides, and is involved in enhancing the root growth (Fávaro et al.,2012).
Although there are other reports describing the antimicrobial action of E. nigrum metabolites against yeast-like fungal human pathogens, the data on their effects against other classes of mycoses-causing fungi, such as dermatophytes, are rather limited. Only one example of growth inhibition of Trichophyton mentagrophytes has been described previously (Mallea et al.,1991). Dermatophytes are a cause of communicative diseases that are acquired from infected animals and humans. The clinical manifestations of the infections that are caused by dermatophytes includepedis and tinea capitis. The most common etiological agents causing dermatophytoses are fungal anamorphs, such as Trichophyton sp. and Paraphyton sp. (Weitzman & Summerbell,1995).
The genus Trichophyton causes the infections among farm animals, mainly in calves and horses, but also in rabbits, sheep, rats, monkeys, cats, and dogs. Human infections might occur after coming in contact with an infected animal. People with impaired immunological system are particularly vulnerable to these pathogens. In contrast, the genus Paraphyton is comprised of anthropophilic, zoophilic, and geophilic species. Among the latter, there are fungi that cause diseases in humans but are not yet reported as pathogenic fungal strains (Weitzman & Summerbell,1995).
It is thus important to search for new antagonists and/or biologically active substances against the dermatophytes. Since higher eukaryotes are the potential hosts of these dermatophytes (Achterman et al.,2011), it is crucial that the biological agents are not toxic against them, and thus in vitro and in vivo toxicity assays should be performed, preferably on mammals, prior to their application (Jorjão et al.,2018). Since the tests on mammals might raise several ethical issues, therefore researchers should develop other eukaryotic models, such as insects for the same. Insect systems have now been extensively used to assess the virulence of fungal pathogens and for in vivo drug toxicity assays due to their low cost, easy culture, and lack of ethical restrictions. Their innate immune system shares many similarities with that of mammals. Among many different insect models, the greater wax moth, Galleria mellonella has been frequently used by the scientific community (Kavanagh & Sheehan,2018).
The major goal of our study was to assess the potential of Epicoccum nigrum as a biocontrol agent against dermatophytes by determining whether it shows in vitro biotic interactions with selected species of dermatophytes, as well as by assessing the effects of its secondary metabolites on the survival and growth of Galleria mellonella larvae.
Material and Methods
The in vitro antagonism between E. nigrum and dermatophytes were studied using the biotic series method described by Mańka and Mańka (1992) and Ogórek and Pląskowska (2011) on PDA (potato dextrose agar; Biocorp) and YPG (10 g/L yeast extract, 20 g/L peptone, 20 g/L glucose, 15 g/L agar) media plates, and described as an individual biotic effect. All the strains used in this study are deposited in the Department of Mycology and Genetics, Institute of Genetics and Microbiology, University of Wrocław, Poland. Two rye isolates of E. nigrum, UP_EPC_31 and UP_EPC_49 (accession numbers KM434173.1 and KM434171.1, respectively), and four species of dermatophytes, including Trichophyton tonsurans Malmsten, isolated from abdominal skin, T. terrestre Durie & D. Frey, isolated from soil, T. mentagrophytes (C. P. Robin) Sabour, isolated from an ambulatory patient, and Paraphyton cookei Ajello KU687323.1, isolated from cave soil, were tested for the interspecies interactions. The fungal inoculates of ca. 4 mm diameter were taken from the ten days old cultures on PDA, and then the mycelium was placed downwards and 2 cm apart in the center of the PDA and YPG plates. Each combination was prepared in four replicates.
Additionally, plates with mycelium of a single fungal species were used as a reference. After inoculation, the plates were incubated in the dark at 24 ± 0.5 °C. Biotic effects of the fungi in the combined cultures were evaluated after 10 days of growth. While evaluating the biotic effects, the surrounding area of one colony that was captured by another fungal species was observed, and then the occurrence of inhibition zone between the two colonies, as well as the reduction in the colony size were considered. The appearance of each effect was scored, and points were summarized according to the scale described by Mańka (1974), and the results are presented in Table 1. The biotic effect induced by a particular fungal species was evaluated as an individual biotic effect (IBE). The positive effect indicates the suppression of pathogen growth, and the negative effect indicates the lack of growth suppression. The effect might be scored with the value of 0, which indicates neutral influence (Mańka,1974; Ogórek & Pląskowska,2011). The size of the zone formed by E. nigrum’s colored metabolites and the size of the inhibition zones created by different E. nigrum isolates were measured. Each measurement was performed in four replicates.
Table 1
The scale of scoring the biotic effects of the Epicoccum nigrum colony on the dermatophyte colony.
[i] The points were given based on the appearance of both the colonies in a Petri dish, as described by Mańka (1974).
Additionally, the toxicity effect of E. nigrum filtrates was examined by using the Galleria mellonella larvae model. Sterile fungal filtrates derived from 14-day-old cultures were incubated at 25 ± 0.5 °C in Sabouraud dextrose broth (peptone 10 g/L and glucose 40 g/L). Thereafter, the caterpillars were treated with 40 µL of the sterile fungal filtrates. Inoculations were performed directly into the hemocoel via the prolegs, by injections using the insulin syringes with 26G needles (Fuchs et al.,2010). Injections were preceded by the disinfection of the puncture sites with 70% ethanol. The inoculations with phosphate-buffered saline (PBS) and Sabouraud dextrose broth were used as the experimental controls. After the injection, the larvae were incubated at 37 ± 0.5 °C, and the viability of the caterpillars was monitored every 24 hr consecutively for 7 days. Caterpillars that were selected for the experiment were in the final instar larval stage (330 ± 30 mg in body weight). Each filtrate was tested on a group of 60 individual caterpillars.
The results were analyzed by one-way analysis of variance (ANOVA) using the software package Statistica version 12.0 (StatSoft Polska, Kraków, Poland). Means of different test conditions were compared using the Tukey’s HSD (honestly significant difference) at α ≤ 0.01.
Results
Overall, both the E. nigrum isolates showed a positive biotic effect towards the tested dermatophytes, with an exception of E. nigrum UP_EPC_31 in the coculture with T. terrestre on YPG (Table 2). The strongest biotic effect was observed for E. nigrum UP_EPC_31 against T. tonsurans on PDA (p T. tonsurans, P. cookei = 0.003661). The same trend was observed in the case of YPG (p T. tonsurans, T. mentagrophytes = 0.009396). In the case of E. nigrum UP_EPC_49, all the interactions were positive, but were not significantly different on both the media plates.
Table 2
The individual biotic effect (IBE) between the strains of Epicoccum nigrum and dermatophytes after 10 days of combined growth on PDA and YPG media plates. The same experiment was performed in four independent replicates.
[ii] 2 For each variant of the experiment, means followed by the same letter are not statistically different at α ≤ 0.01 according to Tukey’s HSD test. Small letters mark differences in the interaction between a particular E. nigrum isolate and the individual dermatophytes species; they refer to column means. Capital letters mark the effect of media on these biotic effects within a given E. nigrum isolate and a given species of dermatophytes; they refer to row means.
The biotic effects were significantly stronger on the PDA plates in comparison to the YPG plates (Table 2). The effect of a culture medium was specifically observed for E. nigrum UP_EPC_31, for which all the interactions varied in a highly significant manner (p PDA, YPG = 0.000327 for T. tonsurans, p PDA, YPG = 0.000349 for T. terrestre, p PDA, YPG = 0.000850 for T. mentagrophytes, and p PDA, YPG = 0.000643 for P. cookei). In the case of E. nigrum UP_EPC_49, statistically significant differences between media were recorded only for T. mentagrophytes (p PDA, YPG = 0.004612) (Table 2).
The results of this study showed that the coculturing of one species with another species, as well as the culture medium, have an effect on the amount of pigments produced by E. nigrum and consequently, the appearance of inhibition zones (Table 3). All the tested dermatophytes stimulated E. nigrum UP_EPC_31 to secrete the colored substances on the PDA plates, with the strongest effect observed for its coculture with T. tonsurans (p T. mentagrophytes, T. terrestre = 0.002184). In contrast, on the YPG plates, this isolate produced colored substances only when it was cocultured with P. cookei. Surprisingly, E. nigrum UP_EPC_49 always synthesized the pigments during the coculture with different dermatophytes, regardless of the medium. Moreover, there was no significant effect of the dermatophyte species on the synthesis of these colored substances by E. nigrum UP_EPC_49 on the YPG media, whereas on the PDA media, this isolate was highly stimulated by T. terrestre (p T. terrestre, T. tonsurans = 0.000670) to produce the pigments. There was also a significant impact of the media on the amount of secreted pigments by E. nigrum UP_EPC_31 (p PDA, YPG = 0.002048 for P. cookei, p PDA, YPG = 0.000291 for T. mentagrophytes, p PDA, YPG = 0.002488 for T. terrestre, and p PDA, YPG = 0.000385 for T. tonsurans), as well as by E. nigrum UP_EPC_49 (p PDA, YPG = 0.000291 for P. cookei, p PDA, YPG = 0.000292 for T. mentagrophytes, and p PDA, YPG = 0.006348 for T. tonsurans), with an exception in the coculture of E. nigrum UP_EPC_49 with T. terrestre. However, in the case of E. nigrum UP_EPC_31, the inhibition zones were only formed on PDA plates in the cocultures with P. cookei (Figure 1), whereas E. nigrum UP_EPC_49 formed the inhibition zones in a coculture with all the dermatophytes on PDA plates (besides T. tonsurans), and only with P. cookei on the YPG plates (Table 3).
Table 3
The ability of Epicoccum nigrum isolates to synthesize colored metabolites and create inhibition zones after 10 days of combined growth with the dermatophytes. A (+) indicates the formation of an inhibition zone, and a (–) indicates the lack of such zones. The indicated values are the average of the values from four independent experiments.
[ii] 2 For each variant of the experiment, means followed by the same letter are not statistically different at α ≤ 0.01 according to Tukey’s HSD test. Small letters mark the effect of a given species of dermatophytes on the synthesis of color metabolites by a given E. nigrum within a particular medium; they refer to column means. Capital letters mark the effect of media on the synthesis of color metabolites by a given E. nigrum isolate in the combined growth with a given species of dermatophytes; they refer to row means.
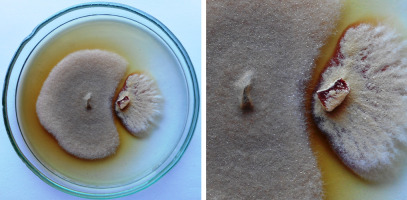
Figure 1
An example of the inhibition zone created by the secondary metabolites secreted by Epicoccum nigrum UP_EPC_31 (left side of the plate) to the PDA medium after 10 days in the paired growth with Paraphyton cookei (right side of the plate); IBE = 5.00.
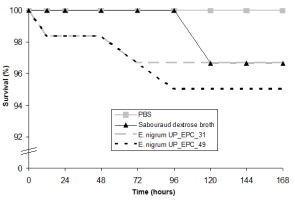
The experiments performed to test the safety of G. mellonella larvae against the E. nigrum filtrates showed that the survival of larvae after 168 hr of incubation with the medium and the E. nigrum UP_EPC_31 filtrate decreased up to 96.7%, and in the case of E. nigrum UP_EPC_49, it decreased up to 95% (Figure 2). Since both, the medium and the secondary metabolites secreted by E. nigrum, reduced the survival rate of G. mellonella larvae to a similarly extent, this reduction in the viability is attributed to the medium, and not to the fungal filtrates.
Discussion
Epicoccum nigrum Link is a cosmopolitan fungus frequently isolated from plants, soil, or water, and is a well-known producer of various secondary metabolites (Sun et al.,2011). The results obtained in our studies confirm the potential application of this species as a biocontrol agent with antimicrobial properties. The antibacterial activity of the secondary metabolites produced by E. nigrum is well documented (Baute et al.,1978; Perveen et al.,2017). Moreover, antifungal properties of this species were also proved against numerous plant, animal, and human pathogens (Mallea et al.,1991). In the present study, we demonstrated the antifungal potential of E. nigrum against dermatophytes, since inhibitory biotic interactions were observed between the E. nigrum isolates and the dermatophytes, including P. cookei, T. terrestre, T. tonsurans, and T. mentagrophytes.
As shown in this study, the type of culture medium is an important factor in estimating fungal interspecies interactions (Ogórek et al.,2016). PDA is a medium preferable for E. nigrum, whereas dermatophytes exhibit better growth on YPG, and thus there were some differences between the strength of the interactions that were dependent on the medium. Epicoccum nigrum is also a well-known producer of colored metabolites and some of them, such as prodiginine, exhibits antimicrobial properties (Perveen et al.,2017). In addition, there is a correlation reported between the secretion of pigments and epicorazine A and B, by this species (Baute et al.,1978). Since the zones created by colored substances and biotic effects were stronger on the PDA medium, we can speculate that medium composition plays an important role in stimulating interactions between E. nigrum and the dermatophytes. PDA is a carbon-rich medium but is deficient in other nutrients (unlike YPG). Such stress conditions might also stimulate the pigment production by E. nigrum (Pradeep et al.,2013), and thus these colored substances might enhance the inhibitory biotic effects towards the dermatophytes (Fatima et al.,2016). As previously reported in the literature, fungi of the genus Epicoccum can also secrete bioactive metabolites with cytotoxic properties, e.g., some terpene metabolites and epicoccamide D (Palacio-Barrera et al.,2019). However, in this research study, we could show that E. nigrum species probably does not produce any cytotoxic substances, since the culture filtrates from E. nigrum strains did not reduce the viability of G. mellonella larvae at a significant level.
In conclusion, this study is the first report describing about the antagonistic interactions between E. nigrum and dermatophytes (P. cookei, T. terrestre, T. mentagrophytes, and T. tonsurans), as well as the effects of its secondary metabolites on G. mellonella, which is an eukaryotic model organism. The results indicate towards the possible application of E. nigrum secondary metabolites for the treatment of skin dermatophytoses. Therefore, in the near future, further studies will help to isolate and identify different secondary metabolites and determine their fungicidal properties.
Handling Editor
Wojciech Pusz; Wrocław University of Environmental and Life Sciences, Poland; https://orcid.org/0000-0003-1531-2739