. Introduction
Snake fruit plants are generally dioecious. For example, Salacca rumphii Wallich ex Blume, which is widespread in Thailand; Salacca sumatrana Becc., that originates from Sumatra; and Salacca zalacca var. zalacca, that originates from Java are dioecious (Anarsis, 2006; Kaputra, 2006; Purnomo, 2013). Salacca zalacca var. amboinensis, however, can be classified as monoecious, because in addition to having sterile male plants that bear only male flowers, the species primarily produces hermaphrodite plants that contain staminate and carpellate reproductive structures in separate flowers but in the same panicle, as in the Bali snake fruit (Smits, 1989).
Like many other aspects of ecosystem function, phenology is influenced by habitat and climate change with both drivers of global change, changing the timing and frequency of phenological events. Here, the process of seeding, flowering, and fertilization also includes phenological events (Morisette et al., 2009). Flower formation and fruit set are strongly influenced by the conditions of the surrounding environment, such as the duration of solar radiation, temperature, and humidity (Fewless, 2006). In the case of Salacca species, the most influential environmental factors for fruit set are air temperature, air humidity, rainfall, and light intensity. Nonetheless, the relationship between phenology and climate is complex (Kyong & Keng, 2012; Ogaya & Peñuelas, 2007). Details of this relationship are yet to be elucidated in snake fruit plants and many other tropical fruit crops. However, understanding the phenology of flowering and fruit set in fruiting snake fruit trees and how this is affected by environmental conditions is important to optimize the production and management of this crop.
Based on interviews with some Sidimpuan snake fruit farmers, it was found that the low production in the off season was partly owing to fruit set failure. Till date, research related to the phenology of flowering and fruit set in the Sidimpuan snake fruit is very limited. Therefore, knowledge of the phenology of flowering and fertilization of the Sidimpuan snake fruit is very important to increase production continuity.
According to Harahap et al. (2013), the Sidimpuan snake fruit plant is not dioecious because panicles contain hermaphrodite flowers with both female and male flowers on the same plant. The Sidimpuan snake fruit is self-pollinating, and the pollination rate is greatly influenced by climatic conditions (Zaimudin, 2002). In optimal weather (rainfall and rainy days: 186.2–294.0 mm/month and 9.2–9.6 days/month, respectively; temperature and light intensity are of minor importance in the climatic conditions of Indonesia), pollination rates are high, but adverse conditions like low rainfall result in the disruption of the pollination process, which in turn affects the fruit set process and fruit production (Hutauruk, 1999). During high rainfall and with more rainy days available (186.2–294.0 mm/month and 9.2–9.6 days/month), the plants can absorb more water, causing higher relative water content (RWC) in the leaves. However, at low rainfall levels, flower development is disturbed, and many inflorescences fail to pollinate and produce fruit (Rai et al., 2010).
The process of growth, development, and production of Salacca crops is also influenced by the availability of macro- and micronutrients, cultivation techniques, and genetic and environmental factors. The agroecological characteristics of the growing site, such as pH and soil N, P, and K content, have been found to affect the production of Sidimpuan snake fruit (Mora, 2014).
This study aimed to determine the phenological events of all flowering and fruit set phases of the Sidimpuan snake fruit until the fruits were ready to be harvested, by recording the number of inflorescences and fruit bunches, the percentage of fruit set, and rainfall and number of rainy days over three flowering periods making up a full year. To determine the potential influence of an internal factor(s) of plants on flowering and fruit formation, leaf N, P, and K content was also analyzed.
. Material and Methods
Study Area and Period
This research was conducted in Palopat Maria village, Padangsidimpuan Hutaimbaru subdistrict, Padangsidimpuan Municipality, Indonesia, located between 01°28′ 19″–01°18′ 07″ N and 99°18′ 53″–99°20′ 35″ E, ±400 m a.s.l., which experiences temperatures of 23–31 °C during the year (Figure 1). This study was conducted between May 2016 and April 2017 on a total area of ±3.5 ha in Sidimpuan zalacca plantations, which implement an agroforestry system based on the agrisilvicultural type of cultivation consisting of a mixture of perennial plants such as rubber, coconut, cocoa, durian, and bamboo with annual vegetables.
Research Methodology
In this study, surveys and descriptive methods were used, consisting of observations and measurements of all observable variables related to flower formation and fruit set at the research location without applying any treatment. The Sidimpuan snake fruit plants used as the samples in this study were mature plants aged 25–30 years.
This study design included two activities:
Survey research. Observations of several quantitative parameters were performed using a completely randomized environmental design because the environmental conditions, plants, and history of cultivation and maintenance of the plants were homogenous. This study followed a non-factorial design with one factor, namely the observation period, which comprised three levels: Period 1 (May–August 2016, abbreviated as May–Aug16), Period 2 (September–December 2016, abbreviated as Sept–Dec16), and Period 3 (January–April 2017, abbreviated as Jan–Apr17), with 10 replications each, so that a total of 30 plants were selected for observation. The observed parameters in this study were the number of inflorescences, number of fruit bunches, and percentage of fruit set.
Morphological observations. Morphological phases of flowers and fruits were observed from the appearance of the inflorescences until the fruit was ready for harvest. Qualitative observations included detailed descriptions of all phases of flowering and fruit formation and these were documented using photographs.
The number of flower bunches and number of fruit bunches were recorded, and N, P, and K content was determined in the leaves, once during each flowering period. Chemical analyses were performed according to the procedures described by Sulaeman and Eviati (2009). Snake fruit leaves from the middle of the leaf midrib (approximately 50 g) from each sample plant were collected, cleaned, and dried in an oven at 70 °C, then blended and sieved with a 0.5-mm mesh size sieve. Total N was determined using the Kjeldahl semimicro method. P and K content was determined using the dry-ash method. The P concentration was measured using a UV–visible spectrophotometer (wavelength range 190–1,110 nm, photometric range 2–3 Abs.; 0–300%, light source 325–370 nm, user selectable). K concentration was determined using a flame photometer.
Analysis of the quantitative data was performed using analysis of variance (ANOVA) and if significant differences were found, data were subsequently analyzed using the least significant difference (LSD) method at the 5% significance level.
. Results
Observation Results: Quantitative Parameters
As shown in Table 1, the highest number of inflorescences was 6.2 bunches in the Jan–Apr17 flowering period, which was not significantly different from the 6.1 bunches observed in Sept–Dec16. However, the number of inflorescences in the two flowering periods was significantly different from the number of inflorescences in May–Aug16 (5.3 bunches). The lowest number of inflorescences occurred during the flowering period in May–Aug16.
Table 1
Total average number of inflorescences and fruit bunches and the percentage of fruit set during the three flowering periods of the Sidimpuan snake fruit.
The highest number of fruit bunches was 4.37 bunches obtained during the flowering period of May–Aug16 as these fruits were a result of flowers produced during the flowering period from Jan–Apr17. These results were not significantly different from the 4.23 fruit bunches in the flowering period Jan–Apr17; however, both results were significantly different from the lowest number of fruit bunches in the flowering period Sept–Dec16 (2.57 bunches).
The highest percentage of fruit set (85.55%) was during the May–Aug16 flowering period, which was significantly different from the percentage of fruit formation in the Jan–Apr17 and Sept–Dec16 flowering periods (69.14% and 44.02%, respectively). The lowest percentage of fruit formation was 44.02% during the Sept–Dec16 flowering period. The number of fruit bunches and flowers formed had considerable influence on the percentage of fruit set (Table 1).
As shown in Table 1, the number of fruit bunches in the Sept–Dec16 period was approximately only 60% of the number observed during the other times of the year and corresponds to a low fruit set compounded by a low number of flowers in the previous period.
The results of the leaf nutrient content analysis are presented in Table 2. The analysis was performed on the leaves of nontreated sample plants because the purpose of observing this variable was to observe the effect of different flowering periods on the leaf nutrient content. Based on LSD analysis, in the May–August and September–December flowering periods, leaf N content was significantly different and higher than that in January–April. The average levels of P and K did not vary significantly throughout the year.
Table 2
Average nitrogen, phosphorus, and potassium content of Sidimpuan snake fruit leaves during the three flowering periods.
| Nutrients | May–August 2016 | September–December 2016 | January–April 2017 |
|---|---|---|---|
| Nitrogen (%) | 2.11b | 2.16b | 1.55a |
| Phosphorous (%) | 0.21a | 0.19a | 0.21a |
| Potassium (%) | 0.43a | 0.44a | 0.46a |
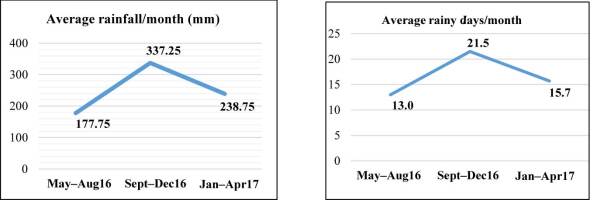
The rainfall and rainy days data were secondary data obtained from the Padangsidimpuan City Agriculture Office. The Sept–Dec16 period was the wettest with both the highest number of rainy days and the highest monthly rainfall (Figure 2 and Figure 3). The May–Aug16 period experienced only approximately half of the monthly rainfall and 60% of the number of rainy days.
Observation Results: Qualitative Parameters (Morphology of Sidimpuan Snake Fruit Hermaphrodite Flowers)
The new inflorescence was formed every 1.0 to 1.5 months and could be observed on the stem around the base of the frond midrib (A in Table 3). All phases of development of inflorescences and fruit bunches occurred on the frond midrib of the snake fruit plant (Table 3) in a circular pattern at each midrib base.
All the phases of the development of inflorescences and fruit bunches are shown in Table 3. In general, flowering and fruit formation followed seven–nine phases consisting of five–six phases of flowering and three–four phases of fruit formation. The flowering phase began with the appearance of a new flower bunch at the base of the leaf midrib every 1.0–1.5 months. The flower development phase lasted for 3.0–3.5 months (Phases A–E). Fruit formation took 3.5–4.0 months from the first set until the fruit was ready to harvest at 5.5–6.0 months after the formation of flowers (Phases F–I).
Table 3
Development phases of flowering and fruit set of Sidimpuan snake fruit (Salacca sumatrana Becc.).
At the beginning of the observations, in Phase A, the inflorescence consisted of one cream colored panicle with a size of approximately 1.0–2.0 cm, and the hermaphrodite flower was not yet seen. In subsequent phases from B to F, one–two panicles were added every 1–2 months, with up to five–seven beige flower panicles in Phase F. The complete development of the panicles is presented in Table 3.
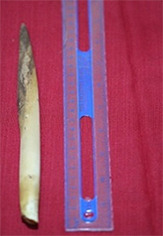
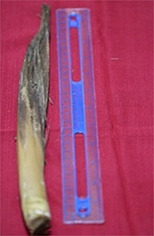
The hermaphrodite flowers bloomed as they entered Phases 5–6. The flower anthesis lasted for ca. 2–3 days, after which they wilted and turned brownish (Figure 3A,B). The blooming hermaphrodite flower was still covered with a sheath, but this split into coarse fibrous strands and fell off post anthesis.
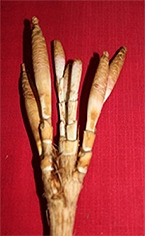
Figure 4
Fruit bunches from Phase F to I of Sidimpuan snake fruit (Salacca sumatrana Becc.). Note: Fa = 6th phase of flowering and fruit set; Fa1–5 = individual flowers in Phase 6: 1 = staminate flower; 2 = pistillate flower; 3 = staminate flower with stamen; 4 = formed fruit; 5 = sheath of a pistillate flower. Fb = 6th phase of flowering and fruit set: 1 = fruit formed in the sheath; 2 = fruit formed with sheath removed. G = fruit bunches in the 7th phase of fruit setting; G1 = fruit diameter 2.0–3.0 cm. H = fruit bunches in the 8th phase; H1 = 9th phase fruit with a diameter of 3.5–4.0 cm. I = fruit ready for harvest (diameter 4.0–6.0 cm).

The Sidimpuan snake fruit with hermaphrodite flowers was self-pollinating. Pollination occurred during Phases D, E, and F when flower bunches were still within their sheaths. No insects or other animals were observed to be involved in the pollination of the flowers, which were enclosed by sheaths.
In the 6th phase, the hermaphrodite flower entered anthesis (Fa in Figure 4). The staminate flower contained stamens (Fa 1 and 3 in Figure 4) and the pistillate flowers comprised ovaries and ovary sheaths (Fa 2, 4, and 5 in Figure 4). After approximately 1 month, the fruits began to appear one by one on the panicles in a bunch. Over the next 4 months, the fruit grew from a diameter of 1.0–2.0 cm to 2.5–3.5 cm, and were 4.5–5.5 cm in diameter when the fruit were ready for harvest (G, G1, H, H1, and I in Figure 4).
. Discussion
The flowering capacity of Sidimpuan snake fruit plants is greatly influenced by the availability of nutrients in the soil and other environmental conditions, in particular, the age of the plant and duration of exposure to low temperatures, which is required for budding and flowering. The different periods of flowering (summer vs. fall) observed in two species by Anton et al. (2014), indicated that different lengths of daytime undoubtedly have an effect on this characteristic. The varying durations of daylight, average daily temperatures, air humidity, and precipitation are well known to modify the diurnal pattern of blooming even in the same species. Our findings are consistent with those reported by Saripudin and Eka (2015) that the phenology of flower emergence in the oil palm is highly dependent on climatic conditions, and the appearance of male flowers is supported by a more humid environment, whereas the phenology of the appearance of female flowers is supported by relatively hot conditions. The same was reported by Gunawan (2007) in mangosteen plants that expressed high individual variation in terms of the number of flowers set in a particular growing season.
The number of flowers produced in a particular growing season depends on many other factors, including individual plant characteristics (Anton & Denisow, 2018). However, the development of flower bunches is influenced by nutrition (Arteca, 1996; Marschner, 1995; Taiz & Zeiger, 2002). In this study, no clear relationship between leaf N content and flower bunch formation was observed. Petraglia et al. (2014) also found no significant correlation between N levels and flowering rates. It is possible that the low leaf N content in the flowering season recorded in Jan–Apr17 in this study could have contributed to the lower number of flower bunches in the subsequent season of May–August. The leaf N content did not appear to have a significant direct effect on fruit set because leaves in this period with the highest percentage of fruit set had the same N content as in the period with the lowest percentage of fruit set. N generally plays a major role in the vegetative phase of plants. While Liferdi and Poerwanto (2011) and Safrizal (2014) found that leaf N levels influenced crop production, P and K are known to be more important for fruit formation.
Fruit bunch formation was related closely to rainfall and the number of rainy days per month. Low rainfall and few rainy days in May–Aug16 correlated with fewer flowering bunches but a higher percentage of fruit set. High rainfall and a high number of rainy days in Sept–Dec16 made little difference to flowering but appeared to reduce fruit set. The rainy season in Jan–Apr17 provided optimal conditions for flowering and subsequently resulted in good fruit set. Similar results were also reported by Rai et al. (2010), Sumantra et al. (2014), and Saputra et al. (2015). The differences in the flowering period could be related to various abiotic factors, such as soil type, water availability, and the ever-changing weather conditions (Elzinga et al., 2007; Fitter & Fitter, 2002).
The development of new hermaphroditic flower buds in the first phase began with a single bunch, as observed in this study. The emergence and growth of new flower bunches occur concurrently with frond midrib growth, and the productive snake fruit plants always have new flower bunches at the base of each leaf midrib (Harahap et al., 2013; Zaimudin, 2002). The development of new sheath-enclosed flower bunches in the next phase is greatly influenced by external and internal factors. Some of these external factors include rainfall, temperature, humidity, and sunlight intensity, whereas the internal factors are nutrient levels in the plant, phytohormones, and leaf water content (Islamy, 2010; Safuan et al., 2013; Singh & Kushwaha, 2006). Eventually, six–eight bunches of flowers and fruits were formed in one sheath of the same panicle. However, at harvest, only approximately four–five bunches remained.
Pollination is essential for fruit set and is the main limiting factor in production (Darmadi et al., 2002; Fatima, 1999; Kriswiyanti et al., 2008; Saripudin & Eka, 2015). Sidimpuan snake fruit plants are self-pollinating and have an apomictic fruit formation process (Hutauruk, 1999). According to Liao et al. (2009) and Holland et al. (2004), hermaphrodite flowers often have a low percentage of fruit set.
Fruit formation is the most critical phase of fruit production and is of vital importance in agriculture. Despite this enormous economic impact, the molecular mechanisms underlying fruit set remain poorly defined (Wang et al., 2009). It is currently accepted that fruit set and development are regulated by the coordinated action of hormones produced in the ovary after pollination and fertilization (Srivastava & Handa, 2005). This is consistent with the observation by Mariotti et al. (2011) that the fruit set is defined as the activation of a developmental process, which converts the ovary into a developing fruit and depends on the interaction among plant hormones.
In summary, this study suggests three likely reasons for the low production of Sidimpuan snake fruit in the September–December period. First, low N levels from January to April may impact flowering in May–August, reducing the fruit set in September–December. If the low N values in January–April are an influencing factor on the low number of flower bunches in the subsequent period, then it could be supposed that applying N fertilizer in the January–April period could result in better flowering rates in May–August, which would then lead to higher snake fruit production in the September–December off-season. Second, if the lower number of flower bunches is in fact owing to the lack of water in the May–August dry season, good irrigation in the dry season (May–August) supposedly would lead to a higher crop in the low crop September–December period. Third, the low fruit set percentage in the September–December off-season means that even fewer of the few flowers from the May–August period would become fruit. This low fruit set percentage appears to be related to the high rainfall during this period. Nevertheless, new flower bunches in Sidimpuan snake fruit plants emerged every 1–2 months at the base of the frond leaf midrib. This suggests that there is great potential for snake fruit plants to fruit throughout the year. In fact, local knowledge suggests that seasonal fluctuations in fruit production were not as pronounced in the past as in recent years. It is hoped that further investigation of the factors revealed in this research may help restore higher off-season productivity in Sidimpuan snake fruit plants.