. Introduction
In the world of flora, the genus Gagea Salisb. (Liliaceae Juss.) comprises from 110 (Tamura, 1998; Xinqi & Turland, 2000a, 2000b) to over 275 (Levichev, 1999; Peterson et al., 2008) species of ephemeral herbaceous plants. It is subdivided into seven (Zarrei et al., 2011) to 14 (Levichev, 2006; Peterson et al., 2008) sections, including sect. Lloydia (Salisb. ex Rchb.) Peruzzi et al., which was considered a separate genus until recently (Peruzzi, 2008). Previously, the genus Lloydia Salisb. ex Rchb. was recognized as separate due to the presence of nectaries at the base of tepals and withering of tepals after anthesis (Heywood, 1980; Tamura, 1998; Xinqi & Turland, 2000b). However, Porsch (1913) with regard to Gagea (without clarification of the species, but not for G. serotina, because the author mentioned it separately) and later Daumann (1970) with regard to Gagea lutea (L.) Ker Gawl. reported perigonal nectaries. Similarly, Peterson et al. (2008) reported that nectaries at the tepal bases were also found in some other Gagea species, although these were very small, visible only on the fresh material, and easily overlooked among herbarium samples. Moreover, molecular studies have shown that Lloydia is not monophyletic and should be considered a section of Gagea, or even spread through several sections (Peterson et al., 2008, Zarrei et al., 2009, 2011).
Most Gagea species are highly decorative ephemeroids that grow in the wild and spread naturally. However, some are planted in artificial green areas (Margelienė, 2009). There are also reports on the use of Gagea in food (Singh et al., 2016; Yeşil et al., 2019) and for its antimicrobial properties (Bader et al., 2018).
Investigations of the vascular anatomy of flowers was introduced by van Tieghem (1871), and is still successfully applied in the investigation of monocots (Dyka, 2018; Novikoff & Kazemirska, 2012; Remizowa et al., 2010; Zalko & Deroin, 2018). Because of its evolutionary conservation, the floral vascular system can serve not only for direct comparison of different taxa, but also for the elucidation of fused organs and analysis of floral evolution and morphogenesis (Joshi, 1940; Novikoff & Jabbour, 2014; Nuraliev et al., 2021; Silva et al., 2016; Sokoloff et al., 2018). Similarly, the principles of the gynoecium vertical zonality were developed by Leinfellner (1950) and Baum (1952), but are still useful in solving phylogenetic and taxonomic issues, in combination with morphological and molecular data (Heigl et al., 2020; Odintsova et al., 2013; Oliveira et al., 2020; Silva et al., 2020). Unfortunately, no investigations on vascularization of the flower or on the vertical zonality of the gynoecium have been performed for the genus Gagea. Therefore, the present study aimed to investigate the peculiarities of floral morpho-anatomy for a few species representing different sections of the genus Gagea to provide new data for further taxonomic and phylogenic studies in this genus.
. Material and Methods
The flower buds of the selected Gagea species were sampled, as shown in Table 1.
Table 1
Sampling material details.
Gagea serotina has been reported to exhibit different sexual phenotypes (Jones & Gliddon, 1999). However, only hermaphroditic flowers were available in our G. serotina material, and these were used for further investigations. Moreover, all other investigated species had hermaphroditic flowers, so it was reasonable to use the same sexual phenotype for comparative investigation in G. serotina.
After fixation, flower buds were passed through the alcohol-chloroform series, embedded in Paraplast Plus (McCormick Scientific, U.S.), and cut into 20-µm-thick cross sections using the rotary microtome MPS-2 (Tochmedpribor, Ukraine). The cross-sections were mounted on microscope slides, dried at 50 °C for 72 hr, and passed through a xylene-alcohol series following the protocol of Barykina et al. (2004). Gagea lutea was stained using 1% safranin solution in 70% alcohol and aqueous 0.5% Astra Blue. Staining of G. reticulata and G. serotina was performed using 1% safranin solution in 70% alcohol and 1% Astra Blue solution in 70% alcohol following the modified Vazquez-Cooz & Meyer (2002) protocol. Slides were then dehydrated in an alcohol-xylene series and mounted using Roti Histokit II (Carl Roth GmbH, Germany). Sections were investigated using an Amplival light microscope (Carl Zeiss Jena, GDR). Microphotographs were captured using an EOS 750D camera (Canon, Japan) adapted to the microscope.
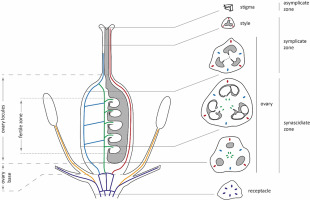
The main parts of the ovary were identified, and their heights were calculated following the suggestions of Odintsova et al. (2013), with minor adaptations. In particular, the height of the ovary base was calculated from the level of perigonium detachment to the level at which the locules appeared (Figure 1). In the investigated Gagea species, delimiting the ovary roof was not possible due to the gradual transformation of the locules into the style channel. Therefore, the height of the locules was calculated from the ovary base up to the level at which the common tri-ray split of the style appeared. Additionally, the height of the fertile zone of the locules was calculated from the level at which the first ovule appeared and up to the last one, at the top of the ovules. Following the principles of Odintsova et al. (2013), the height of synascidiate and symplicate zones was calculated exclusively for the ovary without considering the style and stigma.
Figure 1
A generalized vertical zonality of gynoecium and vascularization of the flower in Gagea. Vascular bundles are marked with colors: violet – undifferentiated and perigonal bundles; orange – staminal traces; blue – lateral carpelar bundles; green – ventral carpelar bundles; red – dorsal carpelar bundles. The dashed line represents the split between locules.
. Results
Flower Morphology and Vertical Zonality of the Gynoecium
The flowers in all investigated species were up to 2.0–2.5 cm long and actinomorphic, and consisted of six tepals (three outer and three inners), six stamens, and a trilocular pistil. The perianth is yellow in all studied species, except for G. serotina, which has white flowers. The pistil is superior, with the ovary elongated-ovate in lateral projection and rounded-triangular in cross-section. The style is thin, rounded-triangular in cross-section, with an apical, short, tri-lobed papillate stigma.
The gynoecium of all investigated species was syncarpous, with a similar organization (Figure 1, Table 2). It consists of three fused carpels with separate locules. At the base, these locules are congenitally isolated (synascidiate zone). However, there is a more or less definable postgenitally fused tri-ray split, which connects the locules (Figure 2B,F). At this level, locules are functionally isolated because of postgenital fusion only (indistinctly symplicate zone). In the upper part of the gynoecium, near the style, locules become connected and form a common cavity, which extends up into the stigma (distinctly symplicate zone). The asymplicate zone is represented by only three independent lobes of the stigma.
Table 2
The height of the ovary structures in Gagea spp.
Numerous (12–21 per locule) anatropous and pleurotropous (oriented approximately perpendicular to the floral axis) ovules are present in the synascidiate and symplicate zones (Figure 2B,D,F). Hence, in all studied species, the placentation is combined – axillar at the ovary base and parietal in the apical part.
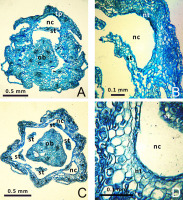
Figure 2
Anatomical details of Gagea lutea (A,B), G. reticulata (C,D), and G. serotina (E,F) flower buds. Arrows indicate the places of postgenital fusion. Abbreviations: an – anastomosis between lateral and ventral vascular bundles; db – dorsal carpelar bundle; lb – lateral carpelar bundle; lc – locule; ne – nectariferous tissue; ov – ovule; st – stamen, tp – tepal; vb – ventral carpelar bundle. Photos are on the level of the synascidiate zone (A,C,E) or on the level of the symplicate zone (B,D,F).
Syntepalous Zone
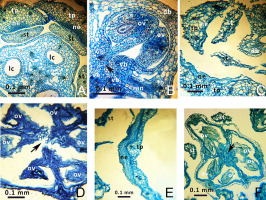
All investigated Gagea species tend to form short (sometimes incomplete) syntepalous zones at the perigonium base; their tepals remain conjoined at a short distance surrounding the ovary. However, stamens become separated very early from the entire ring of tepals and do not take part in the formation of this zone. In G. fragifera and G. pusilla only, the stamens remain conjoined by their edges to the tepals for a short distance, forming nectar-bearing cavities at their bases (Figure 3). The most distinct syntepalous zone, forming a complete ring (approximately 1.5 mm long), was found in G. fragifera.
Perigonal Nectaries
Gagea lutea was found to have nectariferous tissue at the base of all its tepals, consisting of five–six layers of secretory cells (Figure 2A). Similarly, nectariferous tissue consisting of three–four layers of cells was present at the base of all tepals of G. reticulata (Figure 2C), and consisted of one–two layers at the base of all tepals in G. pusilla and G. fragifera. Nectariferous tissue in G. fragifera was less differentiated and very difficult to distinguish (probably due to the early phenological stage at which the flowers were collected). In G. serotina, nectariferous tissue was located differently than in the former species, and was much higher (at the level of the symplicate zone with postgenitally fused carpels). Moreover, this tissue showed well-developed and distinct longitudinal thickening on each tepal (Figure 2E), compared to a flat nectariferous surface observed in the other species.
Floral Vascularization
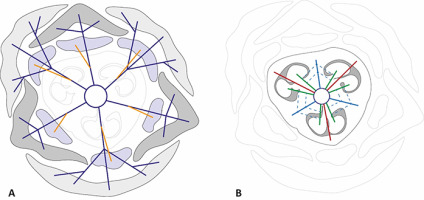
In all studied species, the vascular system in the pedicel are represented by six–eight collateral vascular bundles (Figure 1, Figure 4). In the receptacle, these bundles are divided into 9–12 anastomoses. Next, three bundles on the dorsal radii bent out of the vascular ring and almost immediately subdivided into four independent traces (three supply the outer tepals, and one supplies the stamen). Slightly higher, three bundles on the septal radii also detached from the vascular ring and subdivided into three traces of the inner tepals and stamens. The central bundles extending into the tepals mostly remained intact, while the lateral bundles subdivided. The rest of the vascular bundles in the center of the flower irregularly merged and divided, producing the second ring of 12 carpelar bundles (dorsal, lateral, and ventral). Dorsal carpelar bundles extended without branching into the stigma, while ventral and lateral bundles sporadically anastomosed and supplied the ovules (Figure 2B). Near the style base, ventral bundles bent to the periphery of the ovary and merged with lateral bundles. After that, the lateral bundles continued into the style, but disappeared shortly afterward.
Figure 4
Diagrams of vascularization of perigon and androecium (A) and gynoecium (B) in Gagea. The color code of the vascular bundles is the same as in Figure 1. Dashed lines indicate the anastomoses between lateral and ventral carpelar vascular bundles.
. Discussion
Leinfellner (1950) distinguished three main types of gynoecia: apocarpous (consisting entirely of free carpels and having asymplicate zone only), eusyncarpous (consisting of fused carpels and having synacidiate, symplicate, hemisymplicate, and asymptomatic zones), and hemisyncarpous (consisting of incompletely fused carpels and hemisynascidiate, hemisymplicate, and asymplicate zones). However, it was later shown that this concept does not describe all possible combinations in the gynoecium’s vertical zonality, and 12 main types of syncarpous gynoecia were subsequently suggested (Novikoff & Odintsova, 2008). In particular, the gynoecium of the investigated Gagea has always consisted of synascidiate, symplicate, and asymptomatic zones. Therefore, it does not strictly correspond to either the eusyncarpous or hemisyncarpous type in the sense of Leinfellner (1950), but can be ascertained as a type C, following Novikoff and Odintsova (2008).
Interpretation of vertical zonality of syncarpous gynoecia can be complicated for many reasons, including the fusion of perigon with carpels or different levels of postgenital fusion of carpels (Sokoloff et al., 2018). Hence, it is crucial to reconstruct the primary structure of the gynoecium and determine its secondary transformations. Shamrov (2010, 2012) investigated the vertical zonality of the gynoecium in G. stipitata Merckl. ex Bunge [= G. kunawurensis (Royle) Greuter] and found that it consisted of syncarpous, paracarpous, and apocarpous zones following the terminology of Tahtadzhjan (1980). This terminology does not directly correspond to that proposed by Leinfellner (1950), but can be extrapolated as follows: syncarpous zone = synascidiate; paracarpous = symplicate; and apocarpous = asymplicate. Moreover, Shamrov (2010) noted that the fertile syncarpous zone in G. stipitata is secondary and results from the postgenital fusion of previously paracarpously conjoined carpels. Baum (1949) called such zone secondary syncarpous (secondary synascidiate = originally symplicate) and pointed out that it is not homologous to primary syncarpous (primary synascidiate). In the present study, all investigated species showed congenitally isolated carpels at the base. However, the symptoms of postgenital fusion of the carpels were observed distally (see a dark-colored tri-ray split in the center of the ovary in Figure 2B and F, and disconnected carpels in Figure 2D). Hence, it can be assumed that the primary synacidiate zone in the studied Gagea is relatively short, while most of the ovary, including most of its fertile part, belongs to the secondary synascidiate (originally symplicate) zone. Correct identification of the gynoecium zones is essential for the interpretation of vertical zonality and comparisons between different studies; therefore, postgenital fusion, even if it is difficult to detect, should be considered.
Porsch (1913) reported on Lloydia nectaries in the form of bulges at the base of tepals, and for Gagea, as a flat nectariferous tissue at the base of the inner tepals. Daumann (1970) noted that nectaries in Gagea occur not only on the inner tepals but can also be present at the base of outer tepals, and that they differ in the number of secretory cell layers, depending on the species. In particular, for G. lutea, he reported nectaries consisting of ten cell layers. Here, it was found that nectariferous tissue was present at the base of all tepals of all investigated species of Gagea s. str. (excluding Lloydia), which also corresponds to the findings of Peterson et al. (2008). However, in G. lutea, only five to six layers of nectariferous tissue, and in G. pusilla, only two layers, were found. Moreover, G. fragifera nectaries were extremely small and nearly indistinct.
Hence, we can assume that nectary size and visibility do not necessarily correlate with the species, because nectar production strictly depends on phenology and many other factors, including environmental factors (Bożek, 2019; Dmitruk, 2019; Lovett-Doust & Lovett-Doust, 1988; Nicolson et al., 2007). However, in all of the investigated Gagea s. str., nectaries were similar in their position and shape: they were represented simply by the nectariferous areas of the tepals at their bases. In contrast, G. serotina demonstrated different nectaries: Relatively prominent nectariferous longitudinal thickening was located much higher, while at the base of tepals, there was no nectariferous tissue. Such nectaries were reported for Lloydia by Weberling (1992), who, following Leinfellner (1963), suggested that they are rudimentary peltate structures. Hence, despite the doubtless presence of nectaries in Gagea s. str., as confirmed by the current investigation, they most likely cannot be considered homological to those in G. serotina.
The presence of an extended syntepalous zone and closed cavities at the base of the stamens that serve as nectar reservoirs (Buxbaum, 1937) in G. fragifera is intriguing. This tendency to syntepaly and partial fusion of the lateral sides of stamens with tepals has also been observed in other studied species of the genus. In particular, complete hypanthium and nectar-bearing cavities have been reported for G. lutea by Joshi (1940). The presence of the syntepalous zone allows consideration of the flowers of Gagea as an intermediate between hypogynous and perigynous.
Floral vascularization in all investigated species is quite simple and corresponds to those reported previously for Gagea by Joshi (1940) and Shamrov (2010, 2012). The only important thing to note here is that placentas are supplied not only by ventral bundles, but also by lateral bundles. However, the supply of placentas by lateral bundles, as well as the simple organization of the gynoecium vascular system, is not unique to monocots. It has been sporadically reported for other monocots, including asparagacean Albuca bracteata (Thunb.) J. C. Manning and Goldblatt (Ornithogalum caudatum Aiton) (Novikov, 2008; Tilton & Horner, 1983), asphodelacean Kniphofia uvaria (L.) Oken (Vaikos & Pai, 1982), commelinacean Cochliostema odoratissimum Lem. (Hardy & Stevenson, 2000), and some liliaceans (Sterling, 1974, 1977, 1982; also see other publications of the author). Such a simple organization of the vascular system probably correlates with small flowers with a limited number of ovules; this arrangement does not require the development of additional conductive tissues.
. Conclusions
It was found that all five investigated Gagea species have a similar organization of the flower, vertical zonality of the gynoecium, and floral vascularization. In all cases, flowers are trimerous, have a superior ovary, and have short complete or semicomplete syntepalous zones at the base. Their gynoecium consists of three fused carpels, the synascidiate, symplicate, and asymptomatic zones, which correspond to type C syncarpous gynoecium. It was also found that all investigated Gagea had nectaries at the base of their tepals. However, nectaries in G. serotina are organized and located differently and are probably not homologous to those in other studied species.