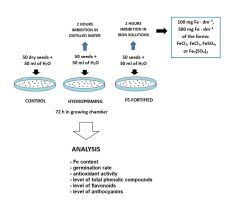
. Introduction
Iron (Fe) is an essential metal indispensable for proper functioning of organisms. In the case of humans, it acts as an oxygen-binding component of haeme groups in haemoglobin and myoglobin and thus plays a crucial role in oxygen transport and storage. Iron constitutes an important element of Fe/S clusters and is engaged in electron transport chains in mitochondria. In addition, as a component of various other proteins, Fe is involved in the processes of replication and repair of nucleic acids, metabolism of neurotransmitters, and defence against pathogens (Abbaspour et al., 2014; Camaschella et al., 2020). In relation to human diet, haeme and nonhaeme Fe can be distinguished. The haeme Fe form is present in meat products and is characterized by higher availability (15–35%). The nonhaeme form is less available (2–20%) although, due to its presence in plant-based products, it is in general the main consumed Fe form. Its uptake is influenced by other dietary components, e.g. phytic acid and polyphenols decrease Fe bioavailability, while ascorbic acid increases its absorption (Abbaspour et al., 2014).
Iron deficiency (ID) in the diet can lead to the development of anaemia, which manifests among others by headaches, fatigue, dizziness, and impaired cognitive functions. A significant decrease in haemoglobin levels may result in lethargy, tachycardia, and heart failure (Aksu & Ünal, 2023). Anaemia negatively affects the life quality of millions of people worldwide, wherein children and women are the most vulnerable groups. According to the World Health Organization (WHO), in the year 2019, anaemia affected over 39% of children under the age of 5 years and 29% of women at the age of 15–49 years (WHO database: https://www.who.int/data, date of access 1.12.2023).
The strategies for combating ID include diversification of diet, supplementation, and fortification and biofortification methods (Malhotra et al., 2021). Fortification refers to the addition of Fe to food products, such as bread, milk, or biscuits. In turn, biofortification aims to increase Fe levels in crops with agronomical methods, traditional breeding practices, or genetic engineering. One of the promising strategies is the biofortification of the seedlings/sprouts of edible plants, such as adzuki bean, mung bean, alfalfa, clover, soybean, radish, and broccoli. Sprouts are attractive components of human diet due to high levels of proteins, minerals, vitamins, and beneficial phytochemicals, such as phenolic compounds, including flavonoids. In addition, the sprouts of plants belonging to the Brassicaceae family contain high levels of glucosinolates transformed to bioactive isothiocyanates (ICT), which are suggested to exert antiproliferation, anticancer, antibacterial, and antioxidant functions. Noteworthy, the process of seed germination is associated with a decrease in the levels of some antinutrient compounds, such as tannins and phytates, which hamper the absorption of minerals, including Fe (Márton et al., 2010; Miyahira et al., 2021).
The aim of the present study was to elaborate methods for simple and cheap enrichment of broccoli seedlings with Fe. Broccoli seedlings were chosen due to their high consumption in the European countries and evidenced health benefits (Márton et al., 2010). In the study, broccoli seeds were imbibed in four different Fe salts (of FeCl2, FeCl3, FeSO4, or Fe2(SO4)3) with Fe applied at two concentrations (100 and 500 mg dm−3). Next, the germination rate, Fe content, antioxidant activity, and the level of total phenolic compounds, flavonoids, and anthocyanins were assessed in 3-day-old sprouts.
. Material and methods
Cultivation and treatment techniques
The seeds of broccoli (Brassica oleracea var. italica), Rabbs cultivar, were surface sterilized for 5 min with 75% ethanol and then for 10 min in a 1% hyperchlorite solution. Next, the seeds were washed for 30 min under running water and imbibed for 2 h in 30 ml of the following solutions: distilled water (hydropriming) or solutions of FeCl2, FeCl3, FeSO4, or Fe2(SO4)3 with Fe at the concentrations of 100 or 500 mg dm−3. Thereafter, the seeds were washed thoroughly under running water and 50 seeds from each variant were placed on glass Petri dishes, 30 cm in diameter, lined with two layers of lignin and one layer of blotting paper. In parallel, 50 dry seeds were also placed on Petri dishes as a control. All variants were watered with 30 ml of tap water. The Petri dishes were transferred to a growing chamber (21–22 °C, 60% humidity) and grown for 72 h in the dark. The assessments of the germination rate, growth, and antioxidant activity were carried out on fresh seedlings. For the other analysis, the seedlings were stored at −80 °C. The design of the study and experimental variants are presented in Figure 1.
Assessment of iron content
For the assessment of Fe content, the 72 h-old seedlings were washed thoroughly, dried for 3 days at 55 °C, and sent to a commercial company (Scallad, Poznań, Poland) for Fe quantification by inductively coupled plasma optical emission spectroscopy (ICP-OES). The measurements were carried out in two independent experimental repetitions.
Measurements of germination rate and seedling growth
The number of germinated seedlings defined as seedlings with 1 mm long radical was calculated after 72 h of germination. The germination rate was calculated according to the formula:
where
GR stands for the germination rate
Ng stands for the number of germinated seeds,
Nt stands for the total number of seeds.
The measurements were carried out in 6–7 independent experimental repetitions.
To assess the toxic effects exerted by the exposure to Fe, growth parameters (root length and seedling fresh weight) were measured after 72 h of growth of the seedlings from the control and hydroprimed seeds and from the seeds exposed to Fe solutions with Fe at the concentration of 500 mg dm−3. The measurements were carried out in four independent experimental repetitions.
Assessment of antioxidant activity
The antioxidant activity was assessed on seedlings germinated for 72 h according to Brand-Williams et al. (1995). Broccoli seedlings (approx. 200 mg) were homogenized in a mortar in 2 ml of 80% methanol, transferred to Eppendorf tubes, and incubated at 37 °C for 2 h. Then the samples were centrifuged at 12,000 rcf for 10 min, and 250 µl of the supernatant was added to a mixture containing 2 ml of 80% ethanol and 250 µl of 2,2-difenylo-1-pikrylohydrazyl (DPPH, Sigma-Aldrich). The absorbance was measured immediately at λ = 517 on a Biomate 3S spectrophotometer (Thermo Fischer Scientific). In the case of the blank, 250 µl of 80% methanol was used instead of DPPH. The measurement was repeated after 10 min long incubation in the dark. The results were expressed as the percentage of DPPH quenching:
where
AT0 stands for absorbance measured before incubation
AT10 stands for absorbance measured after 10 min of incubation
The measurements were carried out in four independent experimental repetitions.
Measurements of the level of total phenolics, flavonoids, and anthocyanins
The level of total phenolic compounds was assessed on seedlings germinated for 72 h according to Díaz et al. (2005). The seedlings (approx. 200 mg) were homogenized in a mortar in 80% methanol, transferred to Eppendorf tubes, and incubated at 70 °C for 15 min. After cooling, the samples were centrifuged at 5,000 rpm for 15 min (Centrifuge 5415R, Eppendorf). The supernatant was transferred to glass tubes and supplemented with 80% methanol to the total volume of 5 ml. Then, 250 µl of the diluted sample was transferred to a new glass tube and supplemented with 3,750 µl of distilled water and 250 µl of Folin-Ciocalteu Reagent (Supelco). After 3 min, the samples were supplemented with 20% Na2CO3 and incubated at room temperature (RT, 20–22 °C) for 2 h. The absorbance of the mixture was measured at λ = 760 (Biomate 3S, Thermo Fischer Scientific). The results were expressed as equivalents of gallic acid. The experiment was carried out in four independent experimental repetitions.
The level of flavonoids was measured in seedlings germinated for 72 h according to Zhishen et al. (1998). The seedlings (approx. 200 mg) were homogenized in a mortar with 1 ml of 80% methanol, transferred to Eppendorf tubes, and incubated at 37 °C for 2 h. Then, the samples were centrifuged at 12,000 rcf at 10 °C for 10 min (Centrifuge 5415R, Eppendorf), and 250 µl of the supernatant was transferred to glass tubes. The supernatant was supplemented with 1,250 µl of distilled water and 75 µl of 5% NaNO3. After 5 min long incubation at RT, 150 µl of 10% AlCl3 was added and the mixture was incubated for another 6 min at RT. Finally, 500 µl of 1M NaOH was added, and the absorbance of the mixture was measured at λ = 510 (Biomate 3S, Thermo Fischer Scientific). The results were expressed as equivalents of quercetin. The experiment was carried out in four independent experimental repetitions.
The level of anthocyanins was measured in seedlings germinated for 72 h according to Sims and Gamon (2002). The samples (approx. 200 mg) were supplemented with a cold mixture of methanol, hydrochloric acid, and distilled water in a ratio of 90:1:1 and stored at 2–4 °C for 24 h. Thereafter, the samples were homogenized using Tissue Lyser II (Qiagen) and centrifuged at 7,000 rpm for 10 min (Centrifuge 5415R, Eppendorf). The absorbance of the supernatant was measured at λ = 529 and 650 (Biomate 3S, Thermo Fischer Scientific).
The level of anthocyanins was calculated on the basis of the formula:
where
AA stands for anthocyanins.
The results were calculated per 1 g of fresh weight.
The experiment was carried out in four independent experimental repetitions.
Statistical analysis
Significant differences were calculated by the one-way ANOVA test using XL Miner Analysis ToolPack (Microsoft). The statistically significant differences between the variants (p = 0.05) were marked with distinct letters in the case of the quantification of the Fe content or by an asterisk (*) in the case of all other analyses, compared to the control.
. Results
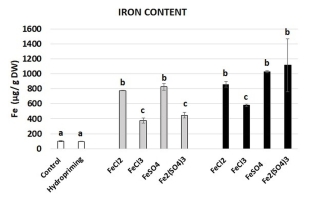
The aim of the study was to evaluate the potential of four Fe salts (FeCl2, FeCl3, FeSO4, and Fe2(SO4)3) to be used for biofortification of broccoli seedlings. The impact of imbibition in Fe solutions on the Fe content in broccoli seedlings was evaluated using ICP-OES. The results are presented in Figure 2. There were no statistically significant differences in the Fe content between the control and seedlings grown from the seeds subjected to hydropriming. On the other hand, the imbibition in the Fe solutions resulted in a significant increase in the Fe content in all of the variants. The lowest (4-fold) increase was noted in the case of the application of FeCl3 and Fe2(SO4)3 with Fe at the concentration of 100 mg dm−3. The most pronounced (approx. 10-fold) increase was observed in the treatment with FeSO4 and Fe2(SO4)3 with Fe at the concentration of 500 mg dm−3.
Figure 2
Iron content in broccoli seedlings grown from the control and hydroprimed seeds (white bars), seeds imbibed in Fe solutions at the Fe concentration of 100 mg dm−3 (grey bars), and seeds imbibed in Fe solutions at the concentration of 500 mg dm−3 (black bars). The results are means of two independent experiments (n = 2) ±SE. Results differing significantly from the each other are marked with different letters.
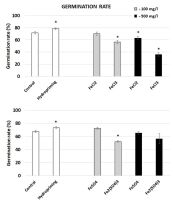
The imbibition of the seeds in distilled water resulted in a slight increase in their germination rate, compared with the control (Figure 3). On the other hand, the imbibition in the Fe solutions had either no effect or an inhibitory effect on the germination rate. In the case of iron chlorides, the application of FeCl2 at the highest concentration (Fe at the concentration of 500 mg dm−3) and FeCl3 in both applied Fe concentrations hampered the germination process. The effect was most pronounced in the treatment with FeCl3 with Fe at the concentration of 500 mg dm−3. In this case, the germination rate decreased by 50%. The imbibition in iron sulphates had milder effects. In this case, germination was inhibited only by the application of Fe2(SO4)3 at the highest Fe concentration.
Figure 3
Germination rate of broccoli seedlings grown from the control and hydroprimed seeds (white bars), seeds imbibed in Fe solutions at the Fe concentration of 100 mg dm−3 (grey bars), and seeds imbibed in Fe solutions at the concentration of 500 mg dm−3 (black bars). The results are means of two independent experiments (n = 6) ±SE. Results differing significantly from the control are marked with an asterisk (*).
To assess whether the imbibition at the highest Fe concentration inhibited growth, seedling fresh weight and root length were measured. The exposure to the Fe solutions had no effect on the growth parameters. Exposure of plants to metals can stimulate the biosynthesis of secondary metabolites (Anjitha et al., 2021). Some of these metabolites can be beneficial for human diet. For instance, diets rich in phenolic compounds can attenuate the progression of neurodegenerative and cardiovascular diseases (Matsumura et al., 2023). Thus, the total antioxidant activity and the levels of total phenolics, flavonoids, and anthocyanins were assessed in the biofortified broccoli seedlings (Figure 4). The hydropriming did not affect any of the parameters, while the imbibition in the Fe solutions had limited effects. No changes were observed in the level of total phenolics (Figure 4B). Some fluctuations in the level of flavonoids were noted, in particular in response to iron sulphates (Figure 4C). However, the changes were not statistically significant. An increase was noted in the antioxidant activity in response to the imbibition in FeSO4 with Fe at the concentration of 500 mg dm−3 (Figure 4A). In addition, the imbibition in FeCl2 with Fe at the concentration of 500 mg dm−3 and in FeCl3 at both applied Fe concentrations resulted in accumulation of anthocyanins (Figure 4D).
Figure 4
Antioxidant activity (A) and the levels of phenolic compounds (B), flavonoids (C), and anthocyanins (D) in broccoli seedlings grown from the control and hydroprimed seeds (white bars), seeds imbibed in Fe solutions at the Fe concentration of 100 mg dm−3 (grey bars), and seeds imbibed in Fe solutions at the concentration of 500 mg dm−3 (black bars). The results are means of four independent experiments (n = 4) ±SE. Results differing significantly from the control are marked with an asterisk (*).

. Discussion
Consumption of sprouts exerts many health benefits. These plant-based food products are rich in proteins, vitamins, minerals, and specific phytochemicals, such as phenolic compounds, including flavonoids and isoflavonoids and, in the case of Brassica sprouts, also in glucosinolates (Márton et al., 2010; Miyahira et al., 2021). The health benefits of sprouts can be further enhanced by their enrichment with essential minerals, e.g. Fe. This aspect is particularly important taking into account the prevalence of anaemia (WHO database: https://www.who.int/data, date of access 1.12.2023). Successful enrichment of sprouts with Fe has been already evidenced e.g. in soybean (Wleklik et al., 2023), adzuki beans (de Oliveira & Naozuka, 2017), radish, broccoli, and alfalfa (Przybysz et al., 2016). In accordance, the results of the present study confirm that imbibition in Fe solutions results in a significant increase in the Fe level in the seedlings (Figure 2). The most pronounced enrichment was observed in the case of application of FeSO4 and Fe2(SO4)3 at the highest Fe concentration, wherein the level of Fe increased nearly 10-fold. Thus, consumption of 10 g of dried biofortified seedlings (corresponding to approximately 40 g of fresh seedlings) can provide 8 mg of Fe and fulfil 100% of the average requirements (ARs) for an adult man and 44% of ARs for an adult woman (EFSA, European Food Safety Authority).
It should however be highlighted that an excess of Fe can exert toxic effects in plants, as observed in rice, wheat, Calliandra (Calliandra calothyrsus), and leucaena (Leucaena leucocephala) showing reduced growth and/or germination rates in response to high Fe concentrations (Aung et al., 2018; El Rasafi et al., 2016; Salim et al., 2021). It is suggested that Fe toxicity is mainly dependent on an excess of reactive oxygen species (ROS), leading to oxidative damage to biomolecules. Moreover, a link has been found between Fe excess and high ethylene levels, which can result in growth inhibition (Li et al., 2024). In the present study, the imbibition in iron chlorites resulted in hampered germination (Figure 3). The application of sulphates had a milder effect, although a significant decrease in the germination rate was noted in the case of imbibition in Fe2(SO4)3 with Fe at the concentration of 100 mg/l. On the other hand, the imbibition in the Fe salts had no effect on the growth of germinated seedlings (Figure S1).
As evidenced by literature data, Fe can act as an elicitor stimulating the biosynthesis of plant bioactive compounds. For example, liquid chromatography mass spectrometry (LC-MS) revealed Fe-dependent accumulation of numerous metabolites in rice. The up-regulation was noted mainly in the case of amino acids and secondary metabolites, including phenolic compounds (Kar et al., 2022). Enrichment of soybean seedlings with Fe resulted in higher content of flavonoids (Wleklik et al., 2023). In the case of broccoli, Fe-enriched sprouts were characterized by elevated levels of ascorbic acids (Przybysz et al., 2016). In the present study, the imbibition in the Fe solutions had limited effects on the antioxidant activity and the level of total phenolic compounds, flavonoids, or anthocyanins (Figure 4). A two-fold increase in the antioxidant activity was noted in the case of imbibition in the solution of FeSO4 at the lower Fe concentration (100 mg dm−3) (Figure 4A). Literature data show that Fe and ROS metabolism are tightly coordinated. In Arabidopsis, exposure to high Fe levels resulted in up-regulation of over one hundred ROS-associated genes, including genes engaged in antioxidant response (Le et al., 2019). Thus, it is possible that also in the present study Fe induced the expression of genes encoding enzymes involved in the biosynthesis of antioxidant compounds, which resulted in enhanced antioxidant activity. In addition, the results of the present study reveal that imbibition in FeCl2 and FeCl3 in general induces accumulation of anthocyanins (Figure 4D). Similarly, a Fe supply resulted in higher levels of total and specific anthocyanins in grape berries (Shi et al., 2017). This was accompanied by induced expression of genes engaged in anthocyanin biosynthesis, including chalcone synthase (CHS) and chalcone isomerase (CHI). It is worth mentioning that phytochemicals can exert dual: beneficial or antinutrient roles. For example, on the one hand, phenolics are perceived as pro-health compounds. Diets rich in phenolics are associated with decreased risk/symptoms of cardiovascular and neurodegenerative diseases (Matsumura et al., 2023). On the other hand, polyphenolics can also hamper the absorption of minerals, including Fe (Abbaspour et al., 2014).
In conclusion, the results of the present study indicate that sulphates constitute a better source of Fe for the enrichment of broccoli seedlings than chlorites. The imbibition of the seeds in FeSO4 and Fe2(SO4)3 resulted in a significantly higher level of Fe in the seedlings, without affecting their germination rate and growth, with the exception of a slight decrease in the germination rate in response to Fe2(SO4)3. In addition, the imbibition in FeSO4 resulted in elevated antioxidant activity, enhancing the pro-health benefits of the enriched seedlings. In turn, the application of FeCl2 and FeCl3 in general resulted in hampered germination and lower Fe content in the seedlings, compared to the treatment with sulphates.
. Supplementary material
The following supplementary material is available for this article:
Figure S1. Root length (A) and fresh weight (B) of broccoli seedlings grown from the control and hydroprimed seeds and from the seeds imbibed in Fe solutions at the Fe concentration of 500 mg dm−3. The results are means of four independent experiments (n = 4) ±SE.