. Introduction
As an academic teacher of plant anatomy, I have always been a little frustrated with the staining methods routinely used in lab classes to demonstrate the anatomical features of plants using fresh sections. I consider hand sections to be a powerful teaching tool, because they give the student a sense of greater independence in completing tasks during lab classes – and thus, greater satisfaction. Most of these staining methods (e.g., Toluidine blue O, safranin-Astra blue, carmine-methyl green) are time-consuming, as they require lengthy rinsing of the excess stain, and their results are often suboptimal due to the simplicity of tools, students’ inexperience in botanical microtechniques and, especially, shortage of time. While experimenting with the use of cheap ready-to-use reagents, offered by one of the commercial companies, for microscopic preparations, I unexpectedly came up with a solution to the above-mentioned problem.
The components of the staining solution proposed here have a long history of routine application in histochemistry. However, Mayer’s mucicarmine is applied mostly in zoological histochemistry, including human pathology, to detect acidic mucopolysaccharides, often in combination with some counterstain: using Google Scholar, the search for references for the phrase “Mayer’s mucicarmine" resulted in ca. 670 records on pathology topics and just a single study on plants (Ginzburg, 1967). Lugol’s reagent is usually used for detection of starch (e.g., Broda, 1971), and in plant tissues treated with the reagent, it stains all other components of the section (cell walls, protoplasts) in various hues of yellow. Characteristically, in the old chlorine-zinc iodide test (=chloroiodide zinc test) in Artschwager’s modification (1921; the modification consisted in the consecutive use of two solutions: solution A equivalent to Lugol’s reagent, solution B: zinc chloride) for differentiation of unlignified from lignified cell walls, the latter remained yellow.
The aim of this study was to determine whether Mayer’s mucicarmine and Lugol’s reagent combined together would be useful in anatomical analyses of plants organs. The staining was proven to be a cheap, fast, and informative staining method, helpful both in teaching and research.
. Materials and methods
The following species and organs were taken for the staining survey: Euphorbia splendens (Euphorbiaceae) stem, Hibiscus rosa-sinensis (Malvaceae) petiole, Homalocladium platycladum (Polygonaceae) stem base, Phalaenopsis sp. (Orchidaceae) aerial root, Rhipsalis sp. (Cactaceae) stem, Schoenoplectus lacustris (Cyperaceae) stem base, Urtica dioica (Urticaceae) stem, and - omitted in the Results due to the recurrency of observations - Aloe arborescens (Asphodelaceae) leaf, Cyanotis somaliensis (Commelinaceae) leaf, and Pilea peperomioides (Urticaceae) petiole and leaf blade. The organs were sectioned fresh with the exception of H. platycladum, Schoenoplectus lacustris, and Urtica dioica, which were FAA-fixed, rinsed repeatedly and stored in 70% ethanol, and briefly immersed in tap water prior to sectioning.
The stock solution of Mayer’s mucicarmine is obtained by heating together water, aluminum chloride, and carmine in a 2:0.5:1 ratio for 2 minutes followed by addition of 100 parts of 50% ethanol (Mayer’s original formula cited by Gray, 1954). However, commercial Mayer’s mucicarmine (Pol-Aura, cat. nr PA-12-98.101) was used in the present study.
The staining protocol imitated conditions of anatomy lab classes, as specified in the Introduction. Organs were hand sectioned using halved commercial razor blades and collected in a watch glass in a few ml of tap water. For the purposes of this study, some ca. 70 µm thick sections were also cut using a vibratome VT1000S (Leica). The best sections were transferred into a diluted solution of Mayer’s mucicarmine: a few drops of the reagent per a few ml of demineralized water (tap water was also sufficient), and the proportions were intentionally approximate, as is usually the case during lab classes. The dilution of the dye was dictated by economy, but staining can also be done in undiluted reagent. The staining lasted as much time as it was necessary to clean the object and cover the slides in a number appropriate to mount the prepared sections (1–3 minutes). Without rinsing, the stained sections were mounted in a drop of water (single staining) or Lugol’s reagent (Broda, 1971) or, alternatively, Lugol’s reagent was “pulled” under the cover slip to replace water (double staining). Next, the sections were examined using a Provis AX70 (Olympus Corporation) light microscope in bright field (BF) or polarization (Po) configuration. Images were saved using a dedicated digital UC90 (Olympus Corporation) camera, operating under the micro imaging software cellSens Standard 1.18 (ISI), as LZW-compressed tiff files of 3384 × 2708 pixels. Digital images were processed using Adobe Photoshop CS7 Extended (Adobe Systems Inc.). Since most sections had uneven thickness, their micrographs were prepared by means of focus-stitching images of several optical “sections” using the Auto-Blend Layers tool. Images of large fragments or of entire sections were obtained by combining several images using the Automatize-Photomerge or the Panorama tool. Finally, the clarity of the images was enhanced using the Levels and/or Curves tools. The final images were prepared for publication in the CorelDRAW®;2020 (Corel Corporation) software.
. Results and discussion
Mayer’s mucicarmine used alone stained most cell walls brightly red (Figure 1). However, unlignified fibers (Figure 1A) and parts of epidermal cell walls (Figure 1B,C) remained unstained or weakly stained. Mucilage was especially emphasized (Figure 1A,B). When Mayer’s mucicarmine was followed by Lugol’s reagent (double staining, Figure 2), the staining differentiated non-lignified cell walls (stained red) from lignified ones (yellow). The mixture also stained starch (initially violet, finally dark brownish-black) and protein-rich cell components, e.g., latex (yellow) or cell nuclei not discernible in the image magnification).
Figure 1
Mayer’s mucicarmine staining results. (A) Hibiscus rosa-sinensis, petiole, BF; (B,C) Rhipsalis sp. stem, BF and Po, respectively. Co - collenchyma, E - epidermis, F - fibers, MC - mucilage cells, arrow - unstained part of epidermal cell wall. Note the smear of red stained mucilage in A and B.
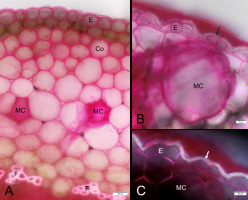
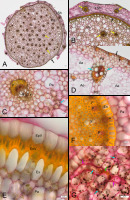
Figure 2
Results of staining with Mayer’s mucicarmine combined with Lugol’s reagent, BF, cross sections except G. (A–C) Homalocladium platycladum stem base sheathed with the dead remnants of leaves; (D) Schoenoplectus lacustris stem base; (E,F) Phalaenopsis sp. aerial root; (G) Euphorbia splendens stem cortex. Ae - aerenchyma, EnV - endovelamen, EpV - epivelamen, En - endodermis, Ex - exodermis, F - fibers, LSh - leaf sheath, P - phloem, Pa - parenchyma, X - xylem, asterisk - passage cell in the endodermis, black arrows - epidermis, yellow arrows - vascular bundle, blue arrows - starch, pink arrows - laticifer’s thick cell wall, green arrow - latex.
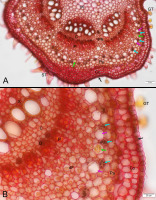
The most satisfying result of the staining with the combined reagents was the effective visualization of the starch sheath in the primary structure of the U. dioica stem (Figure 3), as it facilitated precise determination of the boundary of the primary cortex and the stele, which is often indistinct in dicot herbs.
Figure 3
Detection of the boundary between stem primary cortex and stele – the starch sheath - using Mayer’s mucicarmine combined with Lugol’s reagent, BF, cross section of Urtica dioica upper internode; (B) is a magnified fragment of (A). The starch sheath is sinuous, and its apparent discontinuity results from the asymmetric distribution of the statoliths at the basal cell wall, which is positioned at different levels in neighboring cells. C - fascicular cambium, Co - collenchyma, D - druse cell, dF – area of differentiating pericyclic fibers, GT - glandular trichomes (out of focus in the image), P - phloem, Pa - cortical parenchyma, Pi - pith parenchyma, PR - pith rays, ST - simple setulose trichomes, X – xylem (note yellow staining of the lignified cell wall in differentiated vessels), black arrow - epidermis, blue arrows - statolith starch in the starch sheath cells, pink arrows – laticifers in the outer pericycle, green arrow - latex. Note that most laticifers are seemingly empty.
The anatomical features revealed by the studied method were consistent with the literature, at least at the genus level (Hibiscus petiole: Amri et al., 2019; Rhipsalis stem: Calvente et al., 2008; H. platycladum: Budel et al., 2007; Schoenoplectus: Matushkina et al., 2016; Schweingruber et al., 2020; Phalaenopsis aerial root: Chomicki et al., 2015; Joca et al., 2017; Euphorbia laticifers: Rosowski, 1968; Urtica dioica stem: Gravis, 1884; Metcalfe & Chalk, 1950; Urtica dioica laticifers: Guérin, 1923; Mahlberg, 1993; Metcalfe, 1967; also handbooks on general plant anatomy were consulted: Esau, 1965; Hejnowicz, 2022).
. Conclusions
Staining of sections with the proposed method makes it possible to analyze the anatomy of plants in an easy, quick, and cheap way; additionally, the staining result is aesthetically agreeable. All these advantages are especially important in didactic work, because they give the opportunity to work on sections prepared by students themselves and, through their visual attractiveness, encourage studying plants. The simplicity of the method makes it easily available to microscopy enthusiasts. In addition, due to its informative value, it can facilitate research, especially when supplemented with more specific tests.


