. Introduction
The screening of flora for biological active compounds started in the late nineties (Husein et al., 2014b) and is still being considered nowadays. A. palaestinum Boiss is one of the 26 species of flowering plants of the Arum genus that belongs to the Araceae family (Jaber et al., 2020), which has the highest diversity in the Mediterranean region (Farid et al., 2015). It is widely used as a traditional folk medicinal plant in Arabic and Greek traditions (Máthé & Bandoni, 2021). It has been utilized for treatment of several diseases, such as breast, liver, and colon cancer (Jaradat et al., 2016).
The interest in the A. palaestinum plant is increasing for its bioactive phytochemicals and secondary metabolites that have been discovered and are still being under discovery (El-Desouky et al., 2014; Farid et al., 2015). The leaf extract containing various phytochemical metabolites: flavonoids, phenolic acids, and alkaloids (Abu-Reidah et al., 2015) was reported to have antidiabetic (Hawash et al., 2020) anti-inflammatory (Abu-Qatouseh et al., 2019), and analgesic activities (Qnais et al., 2017) as well as anticancer and antioxidant activities against different cancer cell lines (Jaradat & Abualhasan, 2016). Its extracts were reported to have anti-tumour cytotoxicity towards different cancer cells (Aboul-Enein et al., 2012; Hatmal et al., 2017). Furthermore, its synergistic combinations have been reported to have a promising therapeutic capability against cancer (Booth et al., 2021).
Based on these considerations, we came to the realization that the bioactivity of the flowering parts of A. palaestinum (Figure 1) is yet to be reported. Therefore, we assumed that the examination of bioactivities of A. palaestinum flowering parts would be extremely beneficial and would have a potential application in the treatment of several diseases. In this work, we aimed to study the chemical composition of the non-polar fraction of A. palaestinum spadices and the bioactivities of different extracts of the flowering part. The methanolic extracts of spadices and spathes in addition to the aqueous extract of spadices were evaluated for their anti-inflammatory and anticancer activity against MCF-7, HeLa, Hep-G2, and LX-2 cell lines.
. Material and methods
Chemicals and equipment
Methanol 99% (MeOH), trifluoroacetic acid (TFA), DEME media, fetal bovine serum (99.5%), penicillin-streptomycin (99%), phosphate buffer saline (PBS), trypsin (99.5%), DMSO (99%), Doxorubicin (99.9%), and 5-Fluorouracil (99.9%) were all purchased from Sigma-Aldrich.
Collection of plant material
A. palaestinum (Voucher number BERC-C0064) flowers were collected from Ramallah, West Bank/Palestine during March/April 2021, air dried in a dark place, and preserved at the chemistry labs in Birzeit University, Ramallah/Palestine. The dark purple spadices and spathes were separated, ground, and weighed.
Extraction of plant material
The samples of dried A. palaestinum spadices (41.4880 g) and A. palaestinum spathes (35.3189 g) were extracted three times over night at 4 °C with 400 mL of 10% distilled water H2O in MeOH (v/v; containing 0.5% trifluoroacetic acid, TFA). The combined solutions were filtrated, concentrated under reduced pressure in order to remove the solvent at 35 °C, weighed, and dried completely with Nitrogen to yield 23.3274 g and 14.9934 g respectively (Husein et al., 2014a).
The nonpolar compounds were removed from the A. palaestinum extract by partitioning with 120 mL DW against hexane. The aqueous phase was subjected to Amberlite XAD-7 column chromatography (Hamann, 2006) using 10% distilled water H2O in MeOH (v/v; containing 0.5% TFA) as an eluent to yield 2.0751 g. A sample of dried XAD-7 purified extract was taken for anticancer studies.
A 109.7852 g powder sample of the flowering parts of the plant was soaked twice, each in 620 mL DCM (Devi et al., 2020), basified with ammonium hydroxide (NH4OH) until the solution pH 8–9, filtrated, and concentrated to yield 2.0096 g of crude extract. Another 48.9208 g of the mixture of powdered flowering parts was soaked twice for four hours, each in 300 mL ethyl acetate, basified with NH4OH (until pH 9), concentrated, and dried to yield 0.7352 g of concentrated extract.
GC/MS analysis
The GC/MS analysis (Kaur & Arora, 2015; Zargoun et al., 2020) of volatile compounds was performed on a PerkinElmer Clarus 600 mass spectrometer using a non-polar 5% phenyl methylpolysiloxane TG-5MS capillary column (30.00 m × 0.25 mm × 0.25 mm) in the following conditions: oven temperature program from 80 °C (3 min) to 280 °C at 10 C/min, then isothermal treatment at 280 °C for 3 min. The carrier gas was helium at a flow rate of 1 mL/min. The volume of injected sample was 10 µL of the sample in acetonitrile using a split injection technique (1:10), ionization energy 70 eV, in the electronic ionization mode.
In-vitro cytotoxicity MTS assay and anticancer activity
Cell culture
Four ATCC adenocarcinoma cell lines were used in this study: MCF-7 (human breast cancer cell line), HeLa (immortalized cervical cancer cells), HepG-2 (hepatocellular carcinoma), and LX-2 (normal human hepatic stellate cells).
The cell lines were grown in RPMI-1640 medium supplemented with antibiotics, antimycotics, L-glutamine, and fetal bovine serum. The cells were incubated at 37 °C in a humid chamber containing 5% CO2. A total of 2.5 × 104 cells were seeded in each well of flat 96-well plates and incubated for 72 hours until almost confluent. The cells were then washed with Hank’s balanced salt solution and fresh medium was added. The cells were then treated with different concentrations (250, 500, 2,000 and 4,000 µg/mL) of the three A. palaestinum extracts: Spathe, Spadix, and XAD extracts and incubated as mentioned above.
Cell viability assay
After incubation, the viability of the cells was investigated by the Cell Tilter 96®; Aqueous One Solution Cell Proliferation (MTS) assay according to the manufacturer’s instructions (Promega Corporation, Madison, WI) (Shi et al., 2009). 20 µL of MTS solution per 100 µL of media were added to each well and kept at 37 °C for 2–4 hours. Then, the absorbance was measured at 490 nm with an ELISA reader. The IC50 values of the extracts were determined by plotting the cell viability percentage versus the working concentrations of each extract of the four cell lines separately.
In-vitro anti-inflammatory assay
Blood samples (5 ml) from healthy human volunteers who had not taken any non-steroidal anti-inflammatory drugs (NSAIDS) for 2 weeks were collected. After washing with 0.9% normal saline and centrifuged for 10 min at 2,000 rpm, the volume of the blood was reconstituted as a 40% (v/v) suspension with an isotonic buffer solution (10 mM sodium phosphate buffer pH 7.4, NaH2PO4 (0.2 g/l), Na2HPO4 (1.15 g/l), and NaCl (9.0 g/l)). Standard solutions of acetylsalicylic acid (25–0.195 mg/mL) were prepared by dissolving 100 mg of acetylsalicylic in 2 mL of a hypotonic solution. Blood samples containing the hypotonic solution (154 mM NaCl in 10 mM Sodium Phosphate Buffer at pH 7.4) and the extracts of A. palaestinum spadices, spathes, and XAD were prepared in the same range of acetylsalicylic acid concentrations. Washed red blood cells 10 µm (0.01 mL) were added to each concentration of the control, i.e., the standard hypotonic solution with RBC suspension. The experiment was carried out twice. The mixture was incubated for 10 min at room temperature and centrifuged at 3,000 rpm. The absorbance of the supernatant was measured at 540 nm.
. Results
GC/MS analysis
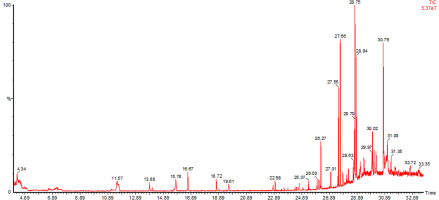
The DCM and EtOAc fractions of A. palaestinum were subjected to GC/MS analysis to identify volatile compounds (Figure 2, Figure S1). A total of twelve major compounds were identified (Table 1).
Table 1
GC-MS analysis of DCM and EtOAc fractions of the flowering part of A. palaestinum.
In-vitro bioactivity assays of A. palaestinum extracts
Anticancer activity of A. palaestinum extracts
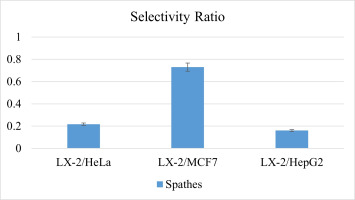
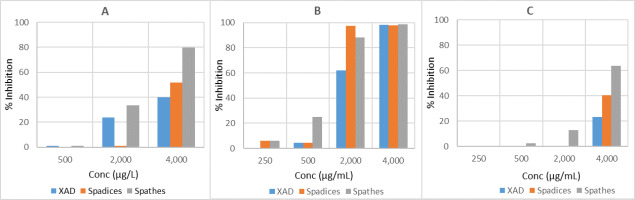
The MTS assay was done on HepG2, HeLa, and MCF7 cells as well as normal cells LX-2 to assess the anticancer effects of A. palaestinum spathe, spadix, and XAD extracts using four concentrations (250, 500, 2,000, and 4,000 µg/mL) (Figure 3, Table S1, Table S2, Table S3). Their IC50 values were evaluated (Table 2) in addition to their selectivity ratios (Figure 4).
Figure 3
The percent inhibition of HeLa (A), MCF7 (B), and HepG2 (C) cancer cell lines using different concentrations of A. palaestinum extracts.
Anti-inflammatory activity of A. palaestinum extracts
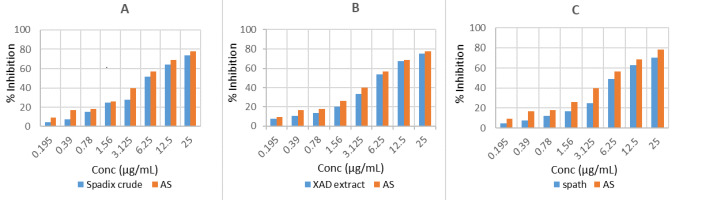
The anti-inflammatory activity was studied using human blood samples collected from healthy volunteers who had not used any NSAIDS for 2 weeks, and it was done as established by Shahriar et al. (2015). The percentage of RBC membrane stabilization or inhibition of hemolysis was calculated by the following equation (Miah et al., 2018) (Figure 5, Table S4, Table S5, Table S6, Table S7).
Figure 5
The percent inhibition of hemolysis (A) A. palaestinum spadice crude extract (B) XAD fraction of A. palaestinum spadices (C) A. palaestinum spathe crude extract.
% Inhibition of hemolysis = 100 ∙ [OD1–OD2/OD1].
OD1 – optical density of hypotonic buffered saline solution alone.
OD2 – optical density of test sample in hypotonic solution.
. Discussion
The taxonomic identification of the plant material was confirmed by Prof. Munir Naser from Birzeit University. The voucher specimen (Arum palaestinum Boiss. Voucher No. BERC-BZ-001) has been deposited at BERC Herbarium, Til, Nablus, Palestine.
GC/MS analysis
Twelve known compounds in the non-polar fraction of A. palaestinum were identified by the GC/MS analysis, which have been recorded to have recognized biological activity. For example, Heptacosane was reported to modulate the activity of P-glycoprotein and exert synergistic effects side by side to the Doxorubicin drug, enhancing its cytotoxic activity against HL-60R cells (Labbozzetta et al., 2022). In addition, 3,5-bis(1,1-dimethylethyl)-Phenol is considered an anticancer agent that is still under study (Rizvi et al., 2014), whereas (Z)-13-Docosenamide was reported to exhibit anti-fungal and anti-tumor properties (Dai et al., 2020). In turn, hexadecanoic acid, a well-known saturated fatty acid, has recently attracted attention and is recently marketed as a health supplement; its methyl ester derivatives are assigned much biological importance, as they have antioxidant, anticancer, antihistaminic, and anti-malarial properties (Melariri et al., 2012). Furthermore, Hexadecanoic acid methyl ester has recently been reported to have antibacterial effects against pathogenic bacteria, such as P. aeruginosa, Klebsiella pneumoniae, and Bacillus subtilis (Shaaban et al., 2021). Squalenes have gained interest as components in cosmetic pharmaceuticals, and they are being under recent ex-vivo studies to evaluate their ability to enhance the physiological functions of different polyphenols in order to increase their skin permeability and improve their antiaging effects (Oliveira et al., 2022).
In-vitro bioactivity assays of A. palaestinum extracts
Anticancer activity of A. palaestinum extracts
Concentrations in the range of 250–4,000 µg/mL of the extracts were used to determine the optimum concentration of the extract exhibiting significant anticancer activity against the cancer cell lines investigated in this study. The specific concentrations are therefore determined through empirical testing and may vary depending on the goals and methodology of the extraction process. Common concentrations used in anticancer studies of plant extracts can be up to 500 µg/mL (Halecki et al., 2022; Ho et al., 2022; Nisa et al., 2022). Testing various concentrations is a standard strategy to determine the optimal concentration of a plant extract, where maximum anticancer activity is achieved while minimizing potential harm to normal cells. Furthermore, initial investigations were carried out to evaluate the cytotoxic impact of plant extracts on normal cells prior to proceeding to subsequent experiments. This precautionary step ensures that the plant extract selectively affects cancer cells without causing substantial harm to healthy cells. In this study, normal cells LX-2 (LX-2 human hepatic stellate cell line) were used and it was found that the extracts used in this study exerted weak or negligible anticancer effects on these human normal skin cells.
Based on the above results, it can be claimed that the spathe extract has the maximum inhibition activity against HeLa cells, which reached about 80% inhibition at 4,000 µg/mL, while the other extracts showed lower inhibition. The extracts of XAD, spadices, and spathes showed significant anticancer activity (more than 98% inhibition) against the MCF-7 cancer cell line at 4,000 µg/mL. These extracts showed moderate to strong inhibitory activity towards the same cancer cells at 2,000 µg/mL, while, low inhibition activity was observed at the concentration of 500 µg/mL. The 4,000 µg/mL concentration showed anticancer activity of all extracts against HepG-2 cancer cells. 63.7% inhibition was exerted by the spathe extract, while 40.5% and 23.3% inhibition was induced by the spadice and XAD extracts, respectively.
All the extracts had higher IC50 values compared to the positive controls Doxorubicin and 5-Fluorouracil against the HeLa and MCF-7 cancer cell lines, which indicates a lower inhibition ability of the A. palaestinum plant extracts compared to the aforementioned drugs. However, only the spathe extract had good activity against the HepG-2 cells in comparison with the other extracts. Noteworthy, the A. palaestinum extracts displayed anticancer properties against the three cancer cell lines. Moreover, these extracts were shown to have weak or negligible anticancer effects on human normal skin fibroblast cells LX-2, which is promising evidence in the field of cancer treatment by plant extracts. Nonetheless, their cytotoxic concentrations are high and controversial. Since several extracts from the aerial parts of this plant have long been claimed to exhibit anticancer and antiproliferative action with very low IC50 values, more research studies are required to validate and strengthen these findings.
Selectivity ratio of the extracts
Based on the fact that the spathe extract yielded the most encouraging results, its effects on normal cells was investigated. Therefore, the selectivity ratio, i.e., % inhibition of the extract of LX-2 to the HeLa, MCF-7, and HepG-2 cancer cell lines, was calculated. The selectivity ratio of the extract was around 0.22 for LX-2 to HeLa, 0.73 for LX-2 to MCF7, and 0.16 for LX-2 to HepG2. If these ratios were compared to those of the positive controls (Dox and 5-FU, which have lower selectivity ratios), the spathe extract could be considered to have possible crucial action against the three aforementioned cancer cell lines. It is important to notice that as the selectivity ratio of a compound or extract increases, it is considered safe compared to compounds with low selectivity factors like Dox and 5-FU.
In this regard, it is widely recognized that certain portions of this plant have anticancer properties. Its ethanolic and ethyl acetate extracts, for example, have been shown to inhibit the growth of MFC-7 cancer cells (El-Desouky et al., 2007; Husein et al., 2014a). Furthermore, a number of its isolated constituents and distinct extracts have been found to exhibit detectable actions against a variety of cancer cell lines, including the cell lines examined in this work: cervix (HeLa), liver (HepG2), and breast (MFC7) carcinoma cell lines (Farid et al., 2015, 2017; Hatmal et al., 2017; Naseef et al., 2017). Similarly, the encouraging findings of the current study, which focuses on the flowering parts of the plant, enhance the bioactive potential of A. palaestinum as a whole medicinal plant.
Anti-inflammatory activity of A. palaestinum extracts
All the A. palaestinum extracts function similarly towards RBC membrane stabilization. They exerted strong activity against lysis with IC50 of 6.25 mg/mL and prevented the hemolysis of the blood cells (induced by the hypotonic solution) up to 75%, compared to acetyl salicylic acid (AS) (78% inhibition at 25 mg/mL). The maximum anti-inflammatory effect was significant at the 25 mg/mL concentration of all the A. palaestinum extracts, which induced more than 70% inhibition. Higher inhibition was achieved by the XAD extract of A. palaestinum. Aqueous extracts could be attributed with increased flavonoid contents (Farid et al., 2015). The anti-inflammatory results of the flowering parts are contrary to the findings for leaf extracts documented by Khalaf et al. (2015), who reported that arum leaves extracts did not exhibit any considerable anti-inflammatory activity. On the other hand, it exerted a unique anti-inflammatory effect, as reported in previous work (Abu-Qatouseh et al., 2019). To the best of our knowledge, this is the first study to investigate the bioactivity of A. palaestinum flowering parts, as all of the previous studies were focused on its aerial parts, especially leaves. In this regard, it should be pointed out that these findings are in agreement with literature reports on the anticancer activity of this plant. Some of its alcoholic and organic, as well as aqueous, extracts have been reported to have anticancer activity against several studied cancer cell lines, including cancer cells that were studied in this work (Azab, 2023).
Extracts from A. palaestinum leaves have more potent anti-cancer effects, compared to plant’s flowering parts, as indicated by the IC50 values found in prior literature. This underscores the necessity for additional research on plant’s spadices and spathes. Moreover, future studies on combinatorial extracts of plant’s aerial and flowering parts are recommended to minimize their active concentrations. It is noteworthy to point out that all extracts were examined for their antibacterial and antifungal activity. No extracts showed any growth inhibition when applied to both Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) and Gram-positive bacteria (Staphylococcus aureus) at all concentrations ranging from 0.78 mg/mL to 100 mg/mL. In the same manner, they exhibited no inhibition activity on the fungi Candida albicans at the same aforementioned concentrations.
. Conclusion
The present study is considered to be the first report on anticancer cytotoxic activities exhibited by extracts from A. palaestinum spadices to provide evidence for its antitumor activities. The cytotoxicity assay of different extracts on the tested cancer cell lines (HeLa, MCF-7, and HepG-2) showed variations in their effects. The examined extracts exhibited concentration-dependent inhibitory activity against the examined cancer cell lines but weak discernible cytotoxic effects. Despite the high cytotoxic concentration, the spathe extracts showed the best inhibitory effects and a relatively high selectivity ratio, which reveals possible crucial action against the cancer cell lines. Nevertheless, the present study showed strong and comparable anti-inflammatory effects of A. palaestinum spadice extracts. Since the GC/MS analysis suggested the potential presence of many bioactive compounds with varying degrees of bioactivity, more investigation is required to validate the bioactivity results.
. Supplementary material
The following supplementary materials are available for this article:
Figure S1. GC/MS chromatogram of non-polar fraction of A. palaestinum spadices (EtOAc extract).
Table S1. % inhibition of HeLa cancer cell lines using different concentrations of the extracts.
Table S2. % inhibition of MCF-7 cancer cell lines using different concentrations of the extracts.
Table S3. % inhibition of HepG2 cancer cell lines using different concentrations of the extracts.
Table S4. Effect of A. palaestinum spadices crude extract towards membrane stabilization.
Table S5. Effect of A. palaestinum spadices/XAD extract towards membrane stabilization.
Table S6. Effect of A. palaestinum spathes crude extract towards membrane stabilization.
Table S7. Effect of acetylsalicylic acid towards membrane stabilization.