. Introduction
Coniferous forests dominate the forest habitat types in Poland and occupy 50.1% of the total forest area (Zajączkowski et al., 2019). Most Scots pine stands are managed (Kubiak et al., 2015) and human activity considerably affects their physiognomy, species structure, and dynamics (Stefańska, 2006). The dominance of Scots pine forest has a significant impact on the species diversity in the forest landscape in Poland (Kubiak et al., 2015). This importance is increased in agricultural landscapes or in areas under other anthropogenic pressures, where the presence of the coniferous forest may allow the survival of many organisms.
Species diversity of epiphytic lichens in a forest community is determined by a number of factors, including stand-related factors. The effect of some has been recognized. Changes in the occurrence and abundance of lichens may appear in response to the impact of the vegetation type (Ardelean et al., 2015; Giordani et al., 2012; Kolanko, 2013; Zarabska, 2009), the species and age structure of the tree stands (Hauck, 2011; Hauck & Spribille, 2005; Kapusta et al., 2004; Kolanko, 2013; Kubiak, 2013; Kubiak et al., 2016; Marmor et al., 2013; Sevgi et al., 2019), quality of available substrata (Kapusta et al., 2004), and the chemical and physical properties of the bark of phorophytes (Hauck, 2011 and literature cited there; Hauck & Spribille, 2005; Kapusta et al., 2004; Kolanko, 2013; Kubiak, 2013; Sevgi et al., 2019). In addition, many studies have proven the influence of microclimatic conditions on the lichenized fungi, including light conditions (Giordani et al., 2012; Hauck, 2011 and literature cited there; Hauck & Spribille 2005; Kapusta et al., 2004; Sevgi et al., 2019), precipitation chemistry, and the input of pollutants (Glanc, 1995; Hauck, 2011 and literature cited there; Hauck & Runge, 2002; Kapusta et al., 2004), especially SO2 and nitrogen pollution (NH3 and NOx) (Hauck, 2011), topographic variables (Ardelean et al., 2015; Sevgi et al., 2019), and stand continuity (Hauck, 2011 and literature cited there).
The lichen biota of Pinus sylvestris in relation to its species richness seems to be well recognized based on local or regional inventories of epiphytes. However, factors determining the occurrence of lichens growing on the bark of this phorophyte are less known. The physicochemical properties of the bark of P. sylvestris affect the composition of the lichen biota (e.g., Kapusta et al., 2004; Zalewska et al., 2004). Marmor et al. (2013) described vertical changes in lichens on the bark of pines. Some species, including rare taxa, can find favorable conditions for their establishment and persistence in higher parts of the trunk and branches of P. sylvestris (Marmor et al., 2013).
We decided to focus on human-related factors in forests and their effect on the species richness and composition of lichens on the bark of P. sylvestris. Forest management is one of the most important factors affecting the persistence of lichens in forest communities (e.g., Kolanko, 2013; Kubiak et al., 2016; Motiejûnaitë & Fałtynowicz, 2005; Zaniewski et al., 2014). Clear cutting, site preparation, artificial replanting, and regular thinning are all part of the intensive management of Scots pine forest in Central Europe (Stefańska-Krzaczek, 2012). The effect of forest management on the species richness of the lichen biota is still poorly documented in Poland (Kubiak, 2013 and literature cited there; Kubiak et al., 2016; Wilkoń-Michalska et al., 1998; Zaniewski et al., 2014). To fill this gap in the knowledge we aimed at recognizing the species diversity on the bark of P. sylvestris considering tree age and forest habitat type in the south-eastern part of Żerków-Czeszewo Landscape Park.
. Material and Methods
Study Area
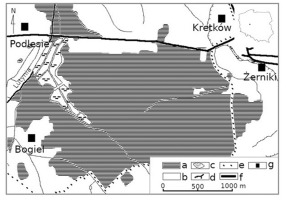
Żerków-Czeszewo Landscape Park is situated in the central part of the Wielkopolska Province, where the Lutynia and Warta rivers join in the Warta valley. The park was established in 1994 and its area now measures 15,800 ha within Miłosław, Nowe Miasto nad Wartą, and Żerków communities (Masztalerz, 2014). The park was established to protect the postglacial relief with special attention to the Warsaw-Berlin ice-marginal streamway (Polish: Pradolina Warszawsko-Berlińska) and culmination of the Żerków Wall (Polish: Wał Żerkowski). These are valuable ecosystems, that in particular include some oak-hornbeam and alluvial forest associations in the Warta valley; rare and protected species of plants, animals and fungi as well as their habitats; and the spatial structure of land considering local landscapes features and valuable culture aspects (Resolution of the Sejmik of the Wielkopolska Province, 2013). The study was conducted in the south-eastern part of Żerków-Czeszewo Landscape Park, in the forest communities occupying approximately 250 ha between the villages of Podlesie, Żerniki, and Ludwinów-Bogiel (Figure 1). Forests in the study area are dominated by Scots pine. The age of these dominant trees did not exceed 98 years. The Lutynia River flows along the western edge of the investigated forest communities. A fishpond complex is located in the western part of the study area, and the road from Żerków to Żerniki runs through the northern part. The agricultural landscape prevails in the surrounding area. In 2015, the mean annual precipitation was 400 mm, and the average annual temperature was approximately 10 °C (Institute of Meteorology and Water Management, 2020).
Figure 1
Study area: a – forests; b – unforested areas; c – breeding ponds and other water reservoirs; d – the Lutynia River and smaller watercourses; e – the border of Żerków-Czeszewo Landscape Park; f – roads; g – villages/settlements.
Żerków-Czeszewo Landscape Park has not been intensively lichenologically explored. Data presented in this article were collected during an inventory aimed at recognition of lichens both within the park and its surroundings, and in this part of the Wielkopolska-Kujawy Lowland. The lichenological survey was conducted in the managed forest typical for the Polish lowlands considering forest habitat types, the dominant tree, and the type of forest management.
Sampling
The study was conducted in 2014 and 2016. Sampled trees were selected randomly in the particular forest habitat types (mixed fresh coniferous forest – MFC; humid mixed coniferous forest – HMC; fresh coniferous forest – FC; mixed fresh deciduous forest – MFD; mixed humid deciduous forest – MHD; fresh deciduous forest – FD). Their locations were distributed within the whole study area. Source data for forest habitat types and the age of the dominant tree were obtained from an interactive forest map of the local forestry authority (Regional Directorate of State Forests in Poznań, 2012). Forest habitat type is one of the main typological systems, especially in practical forestry in Poland (Pielech & Malicki, 2014). This classification is based on the comparison of the fertility and the humidity of the soil, climate, and landform features and geological structure in forest areas (Bańkowski et al., 2003). This study included MFC, HMC, FC, MFD, MHD, and FD. Some select information about their characteristics are given below according to Bańkowski et al. (2003). English translations of the original Polish name of soil types and subtypes were adopted from Kabała et al. (2019). All of the aforementioned forest habitat types are lowland habitat types. MFC includes fresh habitats with mineral soils, under very weak or weak impacts of groundwater. It is mainly found on podzolic rusty soils or typical rusty soils, and much less often on podzolic soils. HMC includes quite poor habitats, under moderate or quite strong influence of groundwater. It occurs mainly on gley-podzolic soils and gley-podzols. In the wetter variant, HMC can be found on, among others, gley and peat soils. FC is characterized by poor, fresh habitats, under very weak or weak influence of waterground. Podzolic soils dominate among the FC soils, with arenosols being less frequent. MFD includes moderately fertile, fresh habitats that are very weakly or weakly affected by groundwater or rainwater. Typical rusty soils, brown-rusty soils, podzolic clay-illuvial soils, and podzolic brown soils are mainly distinquished. Quite fertile and humid habitats, under moderate or quite strong influence of groundwater or rainwater, are typical for MHD. FD includes fertile and fresh habitats, which are very weakly or weakly impacted by groundwater or rainwater. Brown soils and clay-illuvial soils are most often found, while brown-rusty soils, pararendzinas, and black earths are much less common. The proportion of the analyzed forest habitat types reflects their share in the investigated forest communities. Altogether, 201 P. sylvestris trees were sampled (MFC: 154; HMC: six; FC: 24; MFD: nine; MHD: six; FD: two). The age of the pines varied from 26 to 90 years.
Lichens were recorded on the trunk of each tree up to 1.7 m from the ground. When lichen species could not be determined in the field, specimens were collected for further identification in the laboratory using stereoscopic and light microscopy. For the analysis of secondary metabolites in lichen thalli, thin layer chromatography was performed in solvents A and C in accordance with the methods described by Culberson and Ammann (1979) and Orange et al. (2001). The collected specimens are housed in the Department of Agroecology and Bioindication, The Institute for Agricultural and Forest Environment (IAFE) of the Polish Academy of Sciences in Poznań.
Species nomenclature follows Fałtynowicz and Kossowska (2016). The nomenclature of Cladonia coniocraea is based on Diederich et al. (2020). Threatened species categories in Poland those of Cieśliński et al. (2006).
Statistical Analyses
The difference in tree stand age between forest habitat types was tested using the Mann–Whitney test. The Mantel test was used to check the effect of the distance between surveyed trees, based on the geographic coordinates of surveyed research points, the similarity in the number of lichen species per tree, and the similarity in the lichen species composition on trees. The total lichen species richness was assessed with the use of the indicator Chao2-bc (bias corrected). Mao Tau rarefaction curves were used to quantify relationships between the sample size (number of pines surveyed) and the number of lichen species. A generalized linear model (GLZ) was used to quantify the relationships between habitat structure and the number of lichen species. In the model, the explained variable was the number of species per tree, and the habitat variables (predictors) were forest habitat type (FHT) (factorial variable; FHT = MFC or FC), age of the dominant tree (AGE) (numerical variable), and interaction FHT × AGE. The effect of AGE and FHT (MFC vs. FC) on the presence/absence of most common lichen species (i.e., with more than 10 records) was tested with the use of logistic regression. The spatial variation in the lichen species composition (a measure of beta diversity) was analyzed using the Raup–Crick distance as a dissimilarity measure (Vellend et al., 2007). To describe the patterns of spatial turnover of species, we used the first two principal coordinate axes. The next step of beta diversity analysis was testing the difference in community dispersion between fresh mixed coniferous forests and fresh coniferous forests. Datasets of community dispersion (separately for MFC and for FC) consisted of the distances of each sample from a centroid calculated in the principal coordinate space (with first two principal coordinate axes, see above). A permutation F test with 99 permutations was applied to determine if community dispersion was similar in both forest habitat types (Anderson, 2006; Anderson et al., 2006).
The Mann–Whitney test, GLZ, and logistic regression were run with the STATISTICA 12.0 (Statsoft). Rarefaction Mau Tao curves were constructed with the use of PAST 3.22 (Hammer et al., 2001). The values of Chao2-bc indices were estimated with the use of the R package SpadeR (Chao et al., 2016). The assemblage dispersion analysis was performed using R 3.5.2 software (R Core Team, 2018) and VEGAN package (Oksanen et al., 2019).
. Results
Floristic Data
Overall, 26 lichen species growing on the bark of P. sylvestris were found in the study area. Of these species, 11 were crustose (crust.), eight were foliose (fol.), and seven were fruticose (frut.), including Cladonia species. The number of species ranged from one to six per tree (mean, 2.7; median, 3). The most frequent were Cladonia coniocraea (22), Coenogonium pineti (62), Hypocenomyce scalaris (137), Hypogymnia physodes (84), and Lecanora conizaeoides (167). One of the recorded lichens, Evernia prunastri, is a near-threatened species in Poland (Cieśliński et al., 2006). A list of the lichen species found in the investigated area is given in Table 1.
Table 1
Lichen species observed, forest habitat type, morphological form, and number of records assigned in the study.
Effect of Habitat Structure on the Number of Lichen Species
The study sites were categorized according to the forest habitat types: MFC, HMC, FC, MFD, MHD, and FD. To verify the effect of the age of trees and forest habitat types, an analysis considered the lichens growing on the bark of 178 P. sylvestris trees located in MFC and FC, excluding other forest habitat types because of the small number of samples.
The age of tree in MFC forest (31–90 years, median 74 years) was significantly higher compared to FC forest (18–81 years, median 56 years) in the Mann–Whitney test (Z = 3.39, p < 0.001).
The spatial distribution of the investigated trees was aggregated. However, the distance between trees did not affect the lichen species composition (Mantel statistic r = −0.003, p = 0.53) or the number of lichen species per tree (Mantel statistic r = −0.002, p = 0.51).
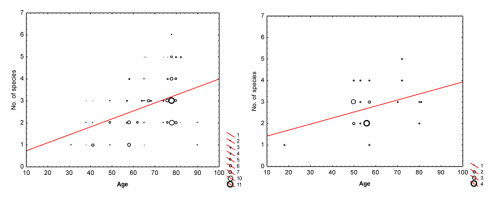
In the GLZ, the number of lichens was positively affected by tree age (Table 2). The effect of pine tree age on lichen alpha diversity (species number per tree) seemed to be independent from the habitat in which tree grew (Figure 2), reflected by the insignificant interaction between the forest habitat type and tree age (Table 2).
Table 2
GLZ relationships between the number of lichen species per tree, forest habitat type (FHT: MFC, FC) and tree stand age (AGE).
| Effects | Coefficient | 95% CI limits | Wald’s statistics | p value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Intercept | 0.24 | −0.39 | 0.87 | 0.57 | 0.4506 |
| AGE | 0.01 | 0.00 | 0.02 | 5.5 | 0.0189 |
| FHT | −0.16 | −0.79 | 0.47 | 0.24 | 0.6230 |
| FHT × AGE | 0.00 | −0.01 | 0.01 | 0.11 | 0.7401 |
Figure 2
Relationships between the number of lichen species (No. of species, y axis) per tree and the age of tree (Age, x axis) in mixed fresh coniferous forests (left) and fresh coniferous forests (right).
Among seven common or moderately frequent species (at least 10 records), the age of phorophytes positively affected the presence of H. scalaris, and marginally positively affected the occurrence of C. coniocraea and H. physodes (Table 3).
Table 3
Logistic regression analysis of the relationship between the presence/absence of three common lichen species on pine and forest habitat type (FHT: mixed fresh coniferous forest vs. fresh coniferous forest) and pine age (AGE).
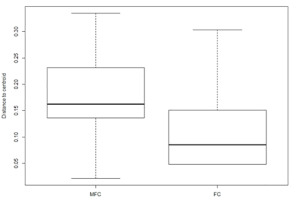
The beta diversity of the lichen community differed slightly between MFC and FC forests. In the similarity space, the locations of lichen communities in FC forests were found in the locations of lichen communities in MFC forests (Figure 3). The findings indicated that although the lichen community in FC forests consisted of the same species sets as in MFC forests, the dispersion of these species in FC forests was lower (Figure 4). The difference was marginally statistically significant in the permutation test for homogeneity of multivariate dispersion (F = 3.11, df = 1, 99 permutations, p = 0.09).
Figure 3
Ordination diagrams of representing a spectrum of lichen communities recorded in mixed fresh coniferous (MFC) forest (black) and fresh coniferous (FC) forest (red) based on principal coordinate analysis, with the use of presence-absence data and Raup–Crick similarity distance.
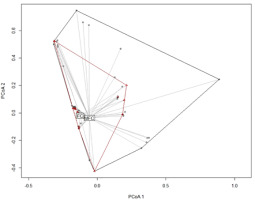
Figure 4
Boxplots of lichen community dispersion (distance between samples and centroid) estimated with Raup–Crick distances in mixed fresh coniferous (MFC) forest and fresh coniferous (FC) forest.
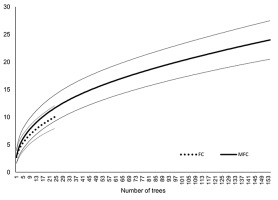
The species richness on the bark of pine in MFC forests was more than three times higher (24 species; Chao2-bc = 38.9, SD = 12.8) than in FC forests (10 species; Chao2-bc = 12.9, SD = 4.0). Therefore, despite the lack of statistically significant differences in the number of lichen species per tree in both studied forest habitat types (alpha diversity), the total species richness (the one considered as gamma diversity) in MFC forests was higher than in FC forests. These results were also confirmed by rarefaction curves in which the total number of species was higher in MFC forests than in FC forests, independent of the number of investigated trees (Figure 5).
. Discussion
Altogether, 26 species of lichenized fungi were recorded on the bark of P. sylvestris in the pine forests in the south-eastern part of Żerków-Czeszewo Landscape Park. Epiphytic lichen biota in Scots pine stands usually comprises few species (e.g., Cieśliński, 1997; Izydorek, 2010; Kolanko & Matwiejuk, 2001; Kubiak et al., 2016; Motiejûnaitë & Fałtynowicz, 2005; Wolseley et al., 2006). However, the lichen abundance can be considerable (e.g., Izydorek, 2010). The physicochemical properties of the bark of P. sylvestris do not favor the development of a rich lichen biota (e.g., Hauck, 2011 and literature cited there; Kapusta et al., 2004; Zalewska et al., 2004). The bark of pine is very acidic with poor water holding capacity and is constantly exfoliated (Zalewska et al., 2004). The higher number of crustose lichen species we recorded, which constituted 42% of the total number of species, could be attributed to the branch structure of pine. Their presence is promoted by dry substrate conditions on the trunk of pine trees caused by loss of precipitation via the branches compared to the trunk (Hauck, 2011 and literature cited there; Sevgi et al., 2019 and literature cited there). Lichenological surveys on Scots pines in Estonian coniferous forests revealed a significant correlation between the total lichen species richness on a tree of pine and species richness up to 2 m. Such information could be helpful in identifying pine trees that potentially harbor the most species (Marmor et al., 2013). Most of the lichenized fungi occurring on pines are common and widespread species, which has been confirmed in lichenological inventories in other parts of Poland (Cieśliński, 1997; Fałtynowicz, 1992; Faltynowicz & Tobolewski, 1989; Izydorek, 2010; Zalewska et al., 2004). The lichen biota is usually slightly specific, with only a few exclusive species (Kubiak et al., 2015). However, according to Marmor et al. (2013), many species, including rare lichens, can occupy a higher part of the trunk and canopy of P. sylvestris and therefore can be omitted when lichenological surveys are conducted in the first two metres above the ground.
We found a significant positive influence of tree age on the species richness on pines, which was independent of the analyzed forest habitat types. Similarly, Izydorek (2010) recorded an increase in the number of species and the lichen cover on trunks with the age of conifer trees and decreased canopy closure. Kubiak et al. (2016) investigated epiphytic lichen biota within managed forests in northern Poland. The results proved that the age of the forest influenced lichen species richness more significantly than the amount of available microhabitat and its heterogeneity. According to Svoboda et al. (2010), tree stand age might be a more important factor in areas that are relatively homogenous with regard to other environmental parameters (climate and air pollution). Our research was not focused on the recognition of the impact of potential factors affecting lichen species richness. We could not compare their effect on the lichen biota. However, apart from some low local emissions, there were no other important sources of air pollution within, and in the vicinity of, our study area. In addition, climatic conditions seemed to be homogenous. Therefore, tree age might be one of the most important factors that significantly determined the species richness of the lichen biota on the bark of P. sylvestris. The physicochemical properties of tree bark change with time (e.g., Glanc, 1965). Marmor et al. (2013) observed the highest number of lichen species up to approximately 10 m from the ground. According to these authors, the smooth peeling part in the upper part of the trunk discourages the presence of lichens (Marmor et al., 2013). This pine bark structure prevails in younger pine stands, and a lower number of species might be associated with this factor. Additionally, changes in microclimatic conditions in the tree stands can also occur with age. Young pine plantations are more shaded, while older tree stands provide more stabilized microclimatic conditions. In the latter, decreasing tree density results in increased canopy openness and improved light conditions on the lower part of the trunk. This favors the development of epiphytic lichen biota [cf. Kubiak et al. (2016) and literature cited there]. However, the dependence of the species richness increase on forest age does not seem so straightforward. In the course of species succession observed on the bark, certain lichen species die out and some new species may appear. A study conducted in a pine forest revealed that the number of species increased in forest stands classified according to their age up to 80 years and then subsequently decreased (Fałtynowicz, 1992; Fałtynowicz, personal communication, October 8, 2020). In our study area, H. scalaris was one of the most frequently recorded species. The presence of this species was strongly influenced by the age of phorophytes. H. scalaris prefers the dry bark of Pinus (Diederich et al., 2020). This species was found to be an indicator for old forests during lichenological inventories in managed Mediterranean black pine forests (Sevgi et al., 2019). Since H. scalaris seems to avoid shaded habitats (Nimis & Martellos, 2017), increased sunlight due to canopy openness and decreasing tree density in older coniferous forests (cf. Kubiak et al., 2016) could have promoted the presence of this lichen in our study area.
The forest habitat type usually reflected by the dominant tree species may affect the diversity of lichens growing on the bark of phorophytes. Although we did not detect differences in the mean number of species per tree (alpha diversity) when comparing forest habitat types, MFC forests were characterized by higher beta- and gamma diversity compared to FC forests. Giordani et al. (2018) conducted biomonitoring surveys. The authors recommended using beta diversity and similarity to analyze temporal and spatial variation in lichen diversity to evaluate anthropogenic impacts. Of note, our analyses were restricted to very similar forest habitat types. Lack of differences in alpha diversity can also be associated with the sole consideration of lichens growing on pines. Tree species influence changes in the occurrence and abundance of lichenized fungi (Hauck & Spribille, 2005; Kapusta et al., 2004; Kolanko, 2013; Kubiak, 2013; Kubiak et al., 2016; Marmor et al., 2013). The increase in diversity is related to the different site conditions accompanied by diversified tree species composition in a forest stand (Hauck, 2011 and literature cited there).
Higher variation in species composition (beta diversity) and higher species richness (gamma diversity) in MFC forests can be associated with a higher microhabitat variability that is caused by the presence of more tree species. This favors the establishment and maintenance of a more diverse lichen biota in MFC forests, including those growing on pines. An increase in the species number could be supported by the occurrence of deciduous trees in coniferous communities (cf. Izydorek, 2010). Diversification of phorophytes, especially the presence of deciduous trees (e.g., oaks, birches, and beeches), in coniferous forests can contribute to the enrichment of the lichen biota (Cieśliński, 1997; Izydorek, 2010; Kubiak et al., 2015; Kubiak et al., 2016 and literature cited there; Sevgi et al., 2019). Presently, spore lichens from sources other than pine phorophytes might also have inhabited the bark of pines in the investigated forest sites. Differences in beta- and gamma diveristy could also be partially related to the age of the tree, which was significantly higher in MFC forests compared to fresh coniferous forests. We proved that the age of trees positively and significantly influenced the species richness on the bark of P. sylvestris.
Since the level of air pollution in the study area does not seem to be detrimental to the species richness of lichens (cf. Svoboda et al., 2010), we expect that human-related forest activities mainly affected the presence of this group of organisms. The anthropogenic impact on the lichen biota in the forest related to forest management can reduce the occurrence, abundance, and vitality of lichens. In the forest stands we studied, P. sylvestris hosted a moderate number of lichenized fungus species. The negative effect of monoculture on the diversity and persistence of the lichen biota, manifested by the disappearance or decrease in abundance of numerous species, has been documented by many authors (e.g., Dingová Košuthová et al., 2013; Fałtynowicz & Tobolewski, 1989; Motiejûnaitë & Fałtynowicz, 2005). Anthropogenic activity in forest ecosystems (i.e., forest management) can affect lichens directly through the destruction of substrates together with lichens growing on them (relevant mainly for epiphytic lichens, less so for terricolous lichens) and/or reduction of the available substrates, such as wood, old trees, and changes in habitat conditions. Old trees provide suitable microhabitats for the occurrence of many epiphytic lichens, including rare species (e.g., Hauck, 2011 and literature cited there, Kubiak, 2013; Kubiak et al., 2016).
As discussed earlier, both species composition of tree stands and changes in the bark properties with the age of phorophytes can be factors that determine the persistence of the lichen biota and its species richness. Age-diversified tree stands enriched the lichen biota growing on pine in the study area, since different species of lichenized fungi can occur in various stages of forest development and some species can be replaced by others. Protection of an area, which strengthens the species protection of lichens, allows the preservation of suitable habitat conditions for the development of lichen biota (cf. Fałtynowicz, 2006).
The study area is located within Żerków-Czeszewo Landscape Park. Effective protection of lichenized fungi and the maintenance of suitable habitats to prevent impoverishment of the lichen biota in the south-eastern part of Żerków-Czeszewo Landscape Park should be primarily based on the careful forest management activities, such as the maintenance of a variety of phorophytes and age-diversified tree stands.