. Introduction
Myxomycetes are eukaryotic, heterotrophic, and cosmopolitan organisms that are classified in the kingdom Amoebozoa (Adl et al., 2012). They occur in all terrestrial ecosystems worldwide. Studies on myxomycetes have been conducted in different plant formations worldwide (Lado & Wrigley de Basanta, 2008; Mitchell, 1995; Ndiritu et al., 2009; Stephenson et al., 1993), including in urbanized areas. Owing to reduced vegetation and the presence of few sites of special natural value, long-term ecological investigations into slime molds in cities have not been conducted frequently, and the majority of publications regarding urban myxomycetes are based on short-term inventory-type studies (Beltrán et al., 2004; Drozdowicz & Wilga, 2002; Hosokawa et al., 2019; Ing, 1998; Orzechowski, 1966; Raciborski, 1884; Rosing, 2009; Wrońska, 1974). Natural sites in cities are affected by anthropopressure. City forests have been degraded or are under pressure from human activity owing to the dense population, housing, recreation, and planting of non-native tree species (Hosokawa et al., 2019). Additionally, city forests are not diverse or rich in species; these ecosystems are poor in microhabitats, which affects the species richness of myxomycetes and other organisms (Hosokawa et al., 2019). Pursuant to the resolution on forests of 1991 (The Forests Act, 1991), urban forests are considered to be protected areas, and the Łagiewnicki Forest is one such protected urban forest. It is one of the largest forest complexes within administrative city borders in Europe, and its most valuable part is protected as the “Las Łagiewnicki” forest reserve (Siciński, 2001). Urban forests provide vital services by having important biocoenotic functions while also contributing to leisure and educational activities (Siciński, 2001).
The occurrence of myxomycetes in the city of Łódź was first reported in the 1970s. Orzechowski noted four taxa from the Łagiewnicki Forest (Orzechowski, 1966). A 2011 study by Ławrynowicz et al. (2011) summarized the knowledge on the biota of slime molds in central Poland, including scarce and occasional records from the Łagiewnicki Forest, and was based on published data and specimens collected over a number of years and deposited in the Herbarium of the University of Łódź (LOD F). The present research aimed to conduct a more systematic and comprehensive investigation of the myxomycete biota of this unique city forest. Thus, the species diversity of myxomycetes was examined, including field observations and qualitative and quantitative analyses. The ecological preferences of the species and phenological data were also recorded. Finally, myxomycete diversity was compared between the forest reserve and the surrounding forest complex.
. Material and Methods
Study Area
The city of Łódź, which is the capital of the Łódź Province, covers an area of 29,320,00 ha. The Łagiewnicki Forest, which covers an area of 1,237 ha (Ojrzyńska, 2001), lies in the northern part of the city (19°29′11.29″ E, 51°50′39.27″ N) (Figure 1). This is the largest green area in Łódź and is a part of the Łódź Hills Landscape Park. The Łagiewnicki Forest is a remnant of an ancient primeval forest that was destroyed over time by settlements and the textile industry (Siciński, 2001). The tree stand is rich in species and has a diverse composition. The forest was planted artificially, but is considered a natural forest and is a remnant of primeval forests. The oldest oak tree stands are over 200 years old (Wrzos, 2001). The dominant tree species are Quercus robur L., Quercus petraea (Matt.) Liebl, and their hybrid (42% of the area); Pinus sylvestris L. (27% of the area); and Betula pendula Roth (20%); other species cover 11% of the area (Wrzos, 2001).
Mesophilic deciduous forests on fertile soils, predominated by oak trees, cover 53% of the Łagiewnicki Forest. Mixed forests and mixed coniferous forests in fertile soils accounted for 37% of the total forest area, while hygrophilous forests form the smallest proportion of forest area (7%) (Kurowski et al., 2001).
Established on November 12, 1996, the “Las Łagiewnicki” forest reserve covers an area of 69.85 ha and is situated in the central part of the forest complex (Figure 1), comprising forest sections and subsections 25c, 25d, 25k, 26a, 26d, 26f, 30a, 30b, and 31a1 (Figure 2) (Andrzejewski & Kurowski, 2001). This forest reserve was established with the aim of protecting a part of the forest with well-developed phytocenoses of subcontinental lowland lime-oak-hornbeam forest Tilio cordatae-Carpinetum betuli Tracz. 1962. Three plant associations were distinguished in the reserve: Potentillo albae-Quercetum petraeae Libb. 1933 n. inv. Oberd. 1957 em. Müller 1991, Calamagrostio arundinaceae-Quercetum petraeae (Hartm. 1934) Scam. Et Pass. 1959, and Tilio cordatae-Carpinetum betuli Tracz. 1962, including its three subassociations, Tilio-Carpinetum stachyetosum, Tilio-Carpinetum typicum, and Tilio-Carpinetum calamagrostietosum (Andrzejewski & Kurowski, 2001).
Figure 1
The location of the Łagiewnicki Forest. Legend: 1 – the city of Łódź on the map of Poland; 2 – the Łagiewnicki Forest on the map of the city of Łódź; 3 – number of forest sections (1–42) in the “Las Łagiewnicki” forest reserve.
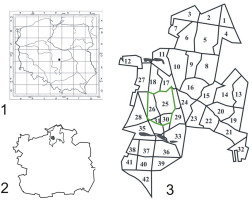
Figure 2
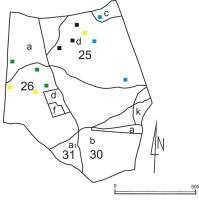
The location of the logs in the reserve: Blue squares – the logs of Betula pendula; black squares – the logs of Carpinus betulus; green squares – the logs of Quercus sp.; yellow squares – the logs of Picea abies; 25, 26, 30, and 31 indicate the forest sections; a, b, c, d, f, and a1 indicate the forest subsections.
Field Observations
Field investigations in the Łagiewnicki Forest were conducted from mid-May to mid-November in 2010–2012. Two research methods were used: the route method and the permanent research plot method. The route method was used in the entire Łagiewnicki Forest from mid-May 2010 to mid-November 2012, with observations being conducted once a month. The permanent observation plots method was used only in the “Las Łagiewnicki” forest reserve. The method was modified, and one designated log was assumed to be a permanent observation plot. Three logs of each of the four tree species [Betula pendula Roth, Carpinus betulus L., Quercus sp., and Picea abies (L.) H. Karst.] were selected for observation in the reserve (a total of 12 logs; Figure 2, Figure 3). This method was used from mid-May to October in 2011 and 2012, with observations conducted every 14 days. The logs differed in size, width, and degree of decomposition. The decomposition of the logs was estimated using the Bobiec scale based on Maser et al. (1979, as cited in Gutowski et al., 2004) (Table 1).
Figure 3
Observed logs: 1, 7, 11 – Betula pendula; 2, 9, 12 – Quercus sp.; 3, 8, 10 – Picea abies; 4, 5, 6 – Carpinus betulus. Photographs by A. Salamaga, 2011-10-08.

Table 1
Analysis of the decomposition of logs using the Bobiec scale based on Maser et al. (1979) (as cited in Gutowski et al., 2004).
All specimens were collected in the field as mature sporangia developed. Only Physarum flavicomum was collected in the form of sclerotium sampled from Betula pendula wood, placed in a Petri dish, and wetted with water. Sporangia were produced after 48 hr. Laboratory examinations were conducted using Nikon SMZ 745T, PZO Warszawa, Wetzlar Hund, and Zeiss Axioskop 2 binoculars and Nikon YS100 light microscope. A permanent slide was prepared using Hoyer’s medium for each specimen. Micromorphological elements were measured at ×100 magnification of the objective lens using immersion oil. The diameter of 20 spores and their ornamentation was measured for each species.
Meteorological data used to illustrate climatic conditions during the study period were obtained from the meteorological station of Łódź Lublinek (Institute of Meteorology and Water Management, 2021).
The scale by Stephenson et al. (1993), was used to determine the occurrence frequency of individual taxa: R – rare (<0.5%; eight records); O – occasional (>0.5%–1.5%; 9–23 records); C – common (>1.5%–3%; 24–46 records); A – abundant (>3%; >46 records).
Collections and permanent microscopic slide preparations were deposited in the MYXO division of the Herbarium Generale at the Institute of Botany, Jagiellonian University (KRA). The nomenclature of myxomycetes was accepted after Lado (2005–2016), that of vascular plants after Mirek et al. (1995), and that of forest associations after Matuszkiewicz (2001).
The myxomycete biota found in the reserve was compared with that in Forest Sections 6 and 7 situated outside the reserve. Only the records found using the route method in 2011–2012 were used for the analysis. Sections 6 and 7 were selected for comparison with the reserve because of their similar size and plant communities:
Size: Łagiewnicki Forest, Sections 6 and 7: 69.44 ha each; “Łagiewnicki Forest” reserve, 69.85 ha;
Plant communities: Łagiewnicki Forest, Sections 6 and 7: Potentillo albae-Quercetum Libb 1933, Calamagrostio arundinaceae-Quercetum petraeae (Hartm. 1934) Scam. Et Pass. 1959 containing Festuca ovina including subassociations: Calamagrostio-Quercetum typicum, Tilio cordatae-Carpinetum betuli including subassociations: Tilio-Carpinetum calamagrostietosum and Tilio-Carpinetum typicum; “Las Łagiewnicki” forest reserve: Potentillo albae-Quercetum petraeae, Calamagrostio arundinaceae-Quercetum petraeae and Tilio cordatae-Carpinetum betuli including its three subassociations: Tilio-Carpinetum stachyetosum, Tilio-Carpinetum typicum, and Tilio-Carpinetum calamagrostietosum (Andrzejewski & Kurowski, 2001).
In addition, previously published data on Oligonema flavidum, Licea minima, Licea variabilis, and Licea pusilla were included in the list of species (Ronikier et al., 2017; Salamaga, 2013).
. Results
In total, 1,561 slime mold samples were collected in the area of the Łagiewnicki Forest (both using the route and plot methods) during the three vegetation seasons. Specimens were assigned to 96 myxomycete taxa (91 species, five varieties) belonging to 26 genera (Appendix S1). Diderma saundersii, Didymium eximium, and Oligonema flavidum are new to the biota of Poland. Arcyria stipata, Hemitrichia calyculata, Oligonema schweinitzii, Physarum flavicomum, and Physarum robustum are on the red list of myxomycetes rare in Poland (Drozdowicz et al., 2006). Stemonitopsis amoena is also referred to as a rare species, as it has been found in a single locality in Poland (Salamaga et al., 2016). Twenty-nine taxa had not been collected previously in central Poland, namely Arcyria major, Arcyria oerstedii, Arcyria stipata, Badhamia foliicola, Badhamia macrocarpa, Badhamia panicea, Comatricha elegans, Comatricha laxa, Craterium aureum, Cribraria persoonii, Cribraria piriformis, Cribraria splendens, Dictydiaethalium plumbeum, Diderma saundersii, Didymium eximium, Didymium nigripes, Didymium serpula, Fuligo septica var. rufa, Hemitrichia calyculata, Licea minima, Licea pusilla, Licea variabilis, Lycogala confusum, Oligonema flavidum, Oligonema schweinitzii, Stemonitis splendens, Stemonitopsis amoena, Symphytocarpus amaurochaetoides, and Trichia botrytis.
According to the accepted classification of species frequency (Stephenson et al., 1993), 57% taxa were rare (55 taxa), 9% were occasional (nine taxa), 27% were common (26 taxa), and 7% were abundant (six taxa).
The greatest number of taxa belong to the genera Physarum (12) and Cribraria (11). One species was noted in each of the following genera: Collaria, Diachea, Leocarpus, Lindbladia, Dictydiaethalium, Metatrichia, Tubifera, and Reticularia.
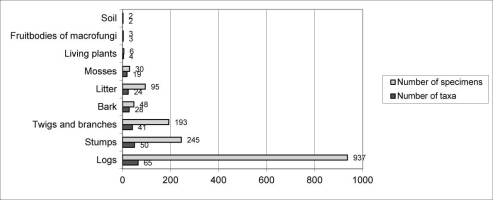
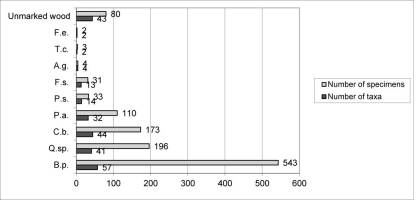
Myxomycetes in the Łagiewnicki Forest were observed on different substrates. However, the greatest number of taxa and specimens were recorded on dead wood and the smallest number were recorded on the soil surface. Dead wood derived from logs was the main substrate (Figure 4). Dead wood of Betula pendula was found on the forest floor, and the greatest number of species and specimens were recorded on this substrate (Figure 5).
Figure 5
Occurrence of myxomycetes on varied tree species: A. g. – Alnus glutinosa; B. p. – Betula pendula; C. b. – Carpinus betulus; F. s. – Fagus sylvatica; F. e. – Fraxinus excelsior; T. c. – Tilia cordata; Q. sp. – Quercus sp.; P. a. – Picea abies; P. s. – Pinus sylvestris.
The survey conducted in the Łagiewnicki Forest using the route method yielded 1,315 specimens, assigned to 95 taxa. Species richness in the “Las Łagiewnicki” forest reserve and the Łagiewnicki Forest was comparable, with a significant difference in the number of recorded specimens: 76 taxa and 422 specimens were found in the reserve, and 77 taxa and 893 specimens in the remaining area of the forest complex. Fifty-nine taxa were common between the reserve and the remaining forest complex. Seventeen taxa were only observed in the reserve, among which were the new recorded species Didymium eximium and Oligonema flavidum, as well as one species from the red list of rare slime molds in Poland, Oligonema schweinitzii (Drozdowicz et al., 2006). Eighteen taxa were found only in the Łagiewnicki Forest, among which was one species from the red list of rare myxomycetes in Poland, Physarum flavicomum (Drozdowicz et al., 2006). The greatest number of taxa in the “Las Łagiewnicki” forest reserve were recorded in 2012 (51) and in the Łagiewnicki Forest in 2011 (61). The highest frequency of occurrence in both areas was demonstrated by the taxa Arcyria cinerea, Ceratiomyxa fruticulosa var. fruticulosa, Ceratiomyxa fruticulosa var. porioides, Lycogala epidendrum, Physarum album, and Stemonitis axifera. The slime molds were recorded on various types of substrates. In both studied areas, the largest number of species and specimens was recorded on logs (forest complex: 50 taxa, 398 specimens; reserve: 55 taxa, 286 specimens), and single taxa were found on herbaceous plants, macrofungal sporocarps, and the soil surface.
In 2011–2012, as a result of observations on selected 12 logs in the reserve, 48 taxa and 246 specimens were noted (Table 2, Appendix S2). After examining each log, a low level of repeatability of taxa and a decline in species diversity in 2012 were observed (40 taxa in 2011, 32 taxa in 2012). The exception was log No. 5, where a comparable number of taxa was found in both years. There were no taxa that occurred on all logs. Ceratiomyxa fruticulosa var. fruticulosa and Lycogala epidendrum were noted on 10 logs each. However, on logs of the four tree species (Betula pendula, Carpinus betulus, Quercus sp., and Picea abies), the following taxa were recorded: Arcyria cinerea, Arcyria obvelata, Ceratiomyxa fruticulosa var. fruticulosa, Ceratiomyxa fruticulosa var. porioides, Fuligo septica, Lycogala epidendrum, and Physarum album. The greatest number of taxa was observed on logs of Carpinus betulus (28 taxa), and the smallest number of taxa was observed on logs of Quercus sp. (17 taxa) (Table 2). As many as 13 taxa were recorded both on logs of Carpinus betulus and Quercus sp., as well as on logs of Carpinus betulus and Picea abies. Only seven taxa were recorded on logs of both Betula pendula and Quercus sp. The degree of wood decomposition was determined for each log (Table 1). Seven taxa occurred across all classes (Table 3). The logs differed according to the degree of decomposition, diameter, and length. The three groups could be distinguished on the bases of the log length (Table 4). The greatest number of taxa was recorded on a 10-m log of Carpinus betulus No. 5. Only half of the taxa were recorded on the longest Carpinus betulus log No. 6, measuring 19 m in length. The smallest number of taxa were found on Carpinus betulus log No. 4, which was also the shortest in length (5 m) and on Quercus sp. log No. 9, which was over three times longer (15.5 m).
Table 2
Myxomycete taxa recorded on logs.
Table 3
Occurrence of myxomycete taxa in different classes of log decomposition.
Table 4
List of taxa on observed logs, including their dimensions, degree of decomposition, and the number of specimens and species.
| Log No. | No. of specimens | No. of taxa | Decomposition degree |
|---|---|---|---|
| Log length over 15 m | |||
| 6 | 19 | 11 | 3 |
| 8 | 16 | 8 | 2–3 |
| 12 | 23 | 14 | 2–3 |
| 9 | 9 | 4 | 2–3 |
| 10 | 21 | 13 | 3 |
| Log length 11–15 m | |||
| 1 | 42 | 18 | 3 |
| 5 | 59 | 23 | 3 |
| 2 | 15 | 6 | 2–3 |
| Log length up to 11 m | |||
| 3 | 24 | 13 | 3–4 |
| 4 | 4 | 4 | 3–4 |
| 7 | 15 | 9 | 3 |
| 11 | 25 | 10 | 3–4 |
A comparative analysis of the biota in the Las Łagiewnicki forest reserve and that in forest sections outside demonstrates that 63 taxa and 324 specimens were found in the reserve and 21 taxa and 27 specimens were found in the Forest Sections 6 and 7. The greatest number of species was found on dead wood formed by logs of different tree species at both sites. Myxomycetes were found to occur on a smaller number of substrates in Forest Sections 6 and 7 than in the reserve.
The Łagiewnicki Forest lies at the norther limit of the geographical range of Abies alba, Fagus sylvatica, Picea abies, and Acer pseudoplatanus L. During the investigations, myxomycetes were found to occur on the dead wood of Picea abies (32 taxa) and Fagus sylvatica (13 taxa).
More than half of the myxomycete species found in the study were differentiated by the substrate colonized: 54 lignicolous taxa were distinguished of which 18 were found in deciduous and coniferous wood, 32 only on deciduous wood, and four only on coniferous wood along with seven foliicolous taxa and two corticolous slime molds. A group of 33 species with a broad range of occurrence was also identified (Table 5).
Table 5
Myxomycete species contribution to individual ecological groups.
The occurrence of myxomycetes depends on the vegetative season. Observations started in mid-May when individual sporangia of Lycogala epidendrum, Lycogala exiguum, and Trichia contorta were noted. However, these were probably well-preserved sporangia persisting from the previous vegetative season. Field observations ended in November each year when the following species were noted: Badhamia capsulifera, Ceratiomyxa fruticulosa var. fruticulosa, Fuligo septica var. septica, Hemitrichia clavata, Metatrichia vesparia, and Lycogala epidendrum. The occurrence of myxomycetes peaked in September 2010, August 2011, and August 2012.
Based on field observations, three phenological groups were distinguished:
Species occurring from early summer to late fall (May–November), which are taxa that do not exhibit phenological preferences. This was the group with the most numerous species, comprising 49 taxa.
Species occurring over 2 months, comprising 21 taxa:
Only July–August (15 taxa): Badhamia foliicola, Collaria arcyrionema, Craterium aureum, Cribraria cancellata var. fusca, Cribraria piriformis, Diderma effusum, Diderma testaceum, Lycogala conicum, Physarum bivalve, Physarum globuliferum, Physarum murinum, Didymium nigripes, Stemonitopsis reticulata, Cribraria persoonii, and Physarum cinereum;
Only August–September (two taxa): Arcyria major and Trichia contorta;
Only September–October (one taxon): Arcyria imperialis;
Only June–July (one taxon): Stemonitis pallida;
Only in August and October (two taxa): Dictydiaethalium plumbeum and Arcyria stipata.
Species noted only in 1 month, comprising 26 taxa:
Only in August (14 taxa): Diderma saundersii, Badhamia panicea, Oligonema schweinitzii, Oligonema flavidum, Craterium leucocephalum, Comatricha pulchella, Symphytocarpus amaurochaetoides, Cribraria persoonii, Badhamia macrocarpa, Comatricha nigra, Cribraria splendens, Symphytocarpus amaurochaetoides, and Didymium serpula;
Only in September (three taxa): Arcyria oerstedii, Licea minima, and Licea variabilis;
Only in October (two taxa): Trichia botrytis and Trichia crateriformis;
Only in November (one taxon): Physarum flavicomum;
Only in July (six taxa): Fuligo septica var. rufa, Didymium melanospermum, Physarum psittacinum, Stemonitopsis amoena, Stemonitis splendens, and Comatricha laxa.
. Discussion
To date, the species diversity of myxomycetes in the Łagiewnicki Forest has been underexplored. Only 20 taxa from the forest were listed (Ławrynowicz et al., 2011; Orzechowski, 1966); four of these were not recorded in the present study: Arcyria ferruginea, Didymium iridis, Lycogala flavofuscum, and Metatrichia floriformis. Slime molds are ephemeral organisms. The lack of records of these species during the research does not mean that they are absent, especially because these species are not considered to be rare and are recorded across different regions in Poland and worldwide (Drozdowicz, 1977, 1992; Härkönen, 1981; Lado & Wrigley de Basanta, 2008; McHugh & Ing, 2012; Mitchell, 1995; Ndiritu et al., 2009; Ronikier et al., 2008; Stojanowska, 2004, 2005).
Although research into myxomycetes occurring in parks and urban forests dates back to the late nineteenth century (Beltrán et al., 2004; Drozdowicz & Wilga, 2002; Hosokawa et al., 2019; Ing, 1998; Orzechowski, 1966; Raciborski, 1884; Rosing, 2009; Wrońska, 1974), published long-term studies investigating the ecology and distribution of myxomycetes in areas of this type are not available. Investigations are usually conducted in nature sites within protection programs or cover entire regions such as mountain ranges (e.g., Beltrán et al., 2004; Erastova et al., 2017; Ronikier et al., 2008). This considerably limits the scope of comparative studies on the biota of the Łagiewnicki Forest.
The Łagiewnicki Forest lies at the northern limit of the occurrence range of Abies alba, Fagus sylvatica, Picea abies (southern part of the range), and Acer pseudoplatanus L. During the investigations, myxomycetes were noted on the dead wood of Fagus sylvatica and Picea abies. Thirteen taxa noted on the dead wood of Fagus sylvatica were also found on the dead wood of other tree species, such as Betula pendula, Quercus sp., and Carpinus betulus. The taxa were assigned to two groups based on their ecology: taxa with a broad range of occurrence (10 taxa) and lignicolous taxa (three taxa). A list of species recorded on dead wood and litter of beech trees in reserves in central Poland was provided by Ślusarczyk in 2010 (Ślusarczyk, 2010). Twenty-six species listed by Ślusarczyk were found in the Łagiewnicki forest on a variety of substrates. Based on the literature, it should be concluded that the species noted by Ślusarczyk are not obligatorily associated with this substrate, as these taxa have been noted not only on dead beech wood, but also on other substrates (e.g., Drozdowicz, 1992, 2017; Kalinowska-Kucharska, 1975; Panek & Romański, 2010; Stojanowska, 2005).
Thirty-two taxa were found on the dead wood of Picea abies. Of these, Arcyria stipata and Stemonitopsis amoena are considered rare species (Drozdowicz, 1977; Härkönen, 1981). Taxa belonging to two ecological groups were also noted: taxa with a broad range of occurrence (15) and lignicolous taxa (17). Five species were found only on the dead wood of Picea abies: Lindbladia tubulina, Stemonitis splendens, Stemonitopsis amoena, Trichia botrytis, and Cribraria tenella. However, these taxa have also been reported from a variety of substrates in other parts of Poland (Drozdowicz, 2017; Panek & Romański, 2010) and therefore cannot be considered a characteristic of this substrate type.
Over the years, the following ecological groups have been distinguished – corticolous, lignicolous, foliicolous, musicolous, fungicolous, coprophilous, epiphyllic (occurring on living leaves), succulenticolous (developing on dead parts of succulents), and epiflorous species (developing between living and dying parts of inflorescences). Four categories – lignicolous, corticolous, foliicolous, and coprophilous species – are considered to be of special importance as they form distinct groups that are easily distinguishable from one another (Ing, 1994; Novozhilov et al., 2000; Rollins & Stephenson, 2011).
Three ecological groups were identified in the Łagiewnicki forest: lignicolous, foliicolous, and corticolous species. An additional group of species with a wide range of occurrence on different substrate types was identified. Lignicolous myxomycetes were the most abundant group (54 taxa) (Table 5); most of these were observed on the dead wood of different species of deciduous and coniferous trees, but 16 taxa were noted only on wood of a specific tree species: Cribraria persoonii, Cribraria splendens, Physarum flavicomum, Physarum psittacinum, and Trichia contorta were noted only on Betula pendula; Cribraria tenella, Lindbladia tubulina, Stemonitis splendens, Stemonitopsis amoena, and Trichia botrytis were noted only on Picea abies; Symphytocarpus amaurochaetoides and Trichia crateriformis were noted only on Carpinus betulus; and Licea pusilla, Licea variabilis, and Badhamia macrocarpa on Quercus sp. The aforementioned species can be classified as lignicolous myxomycetes, but they are not obligatorily associated with the dead wood of the tree species on which they were observed. For instance, Trichia contorta has been noted on the wood of Fraxinus excelsior (Iršėnaitė et al., 2013), Stemonitis splendens has been noted on the wood of Betula sp. (Panek & Romański, 2010), and Stemonitopsis amoena has been noted on the wood of Pinus sylvestris (Salamaga et al., 2016) and on a log of Ulmus sp. (Adamonyte & Kastanje, 2017).
In addition to dead wood, litter is often colonized by myxomycetes. In the Łagiewnicki Forest, this ecological group was represented by seven taxa (Table 5). A part of the life cycle of Oligonema is strongly dependent on water; therefore, the occurrence of a species of this genus is not associated with the substrate type but with a specific habitat that is waterlogged or periodically moist (De Haan et al., 2004). The other taxa are noted very often on this substrate type (Drozdowicz et al., 2003; Lado et al., 2013; Müller et al., 2007; Rollins & Stephenson, 2012; Stephenson, 1989; Takahashi, 2013; Takahashi & Hada, 2012), although they have been sporadically observed on dead wood (Drozdowicz et al., 2003; Müller et al., 2007). Species developing in the litter form fine and brittle sporangia that are difficult to notice, making field observations arduous and time-consuming. Moist chamber cultures of litter sampled in the field are used to investigate litter-inhabiting myxomycetes (De Haan et al., 2004; Stephenson, 1989).
Myxomycetes that occur on the bark of living trees usually form small sporangia. Moist chamber cultures were also used for examination. Bark was sampled from living trees and cultured in Petri dishes. This method was not used in studies in the Łagiewnicki Forest, but during field observations, yellow aggregates resembling sclerotium were seen on Betula pendula logs. Samples were removed, placed in a Petri dish, and wetted with water. After 48 hr, mature sporangia appeared and were classified as Physarum flavicomum. However, two taxa, Badhamia foliicola and Fuligo septica var. rufa, were found on living bark and were classified in to a separate group. Both species form sporangia visible to the unaided eye and have been noted in the field on different substrate types, mainly on dead wood (Castillo et al., 2009; Takahashi & Hada, 2012).
A group of 33 taxa colonizing different substrate types was distinguished in the field (Table 5). This group includes all taxa classified as abundant on the frequency scale: Arcyria cinerea, Ceratiomyxa fruticulosa var. fruticulosa, C. fruticulosa var. porioides, Lycogala epidendrum, Physarum album, and Stemonitis axifera. Species in this group were noted mainly on the dead wood of different species. Single myxomycetes were noted on bryophytes, fruitbodies of macrofungi, soil, and living parts of herbaceous plants. Bryophytes and fruitbodies of macrofungi from which myxomycetes were noted were found to overgrow logs.
Myxomycetes tend to occur seasonally, although their phenology varies across different climatic zones (Ing, 1994). In Poland, investigations into myxomycetes (excluding nivicolous species) usually start in May and end in either October or November depending on weather conditions. In the Łagiewnicki Forest, new mature sporangia were almost entirely absent in May and only remnants of sporangia from the previous vegetative season were observed in May 2010 and 2011. Three groups of taxa were distinguished in the study based on their phenology: those occurring throughout the entire vegetative season, those occurring over 2 months, and those found only in 1 month throughout the entire study. No preference for specific months of the vegetative season was observed in over 50 percent of the taxa. Only single records of species occurring in a specific month were found, and their preferences could not be determined. Mean temperature values were similar during the vegetative season in 2011 and 2012, with the highest temperature values recorded in June and August in 2011 and July in 2010 and 2012. The highest precipitation was noted in 2010, with values peaking in May. The mean precipitation in 2011 and 2012 was similar. Precipitation peaked in July 2011 and June 2012 (Figure 6).
Figure 6
Occurrence of myxomycetes in the vegetative season in 2010–2012 in view of the precipitation and temperature (meteorological data).

Owing to the use of the route method in the reserve and forest, it was possible to review many microhabitats that could be settled by slime molds in these areas. The species diversity of both species was similar. However, differences were noted when comparing the reserve area with the selected Forest Sections 6 and 7 for the same surface. First, in the reserve, the number of recorded specimens is several times and the number of taxa is 3 times greater than those in the forest, because the amount of dead wood in the reserve is much greater than that in the selected forest sections. The same preferences apply to dead wood and its various forms.
Myxomycetes are a specific and difficult group in field research. Most species inhabit dead wood, especially logs that provide good moisture conditions. As indicated by observations of 12 selected logs over two vegetative seasons, the species diversity decreased in the second year of observations. Fewer new species were recorded or not recorded at all. Additionally, the reoccurrence of the same species was low in the second year of the study, while mean temperature and precipitation values were similar between the years. It is difficult to determine the reason for this change as the occurrence of slime molds depends on many factors. It must be highlighted that the investigations, including specimen collection, were not disruptive and sporangia were not gathered in their entirety. It is also possible that the species may have occurred between the observation periods. Some species are characterized by cyclicality during sporangium production. A comparison of the biota of logs of the four tree species showed that the greatest number of the same species occurred on Betula pendula and Quercus sp., and the smallest number of the same species occurred on Quercus sp. and Picea abies. A common feature of all established research areas was the relatively low repeatability of recorded taxa in the second year of research, with similar temperature and precipitation values. It is difficult to determine the reason for this feature because the occurrence of slime molds depends on many factors. It is possible that the lack of these species was not indicative of their nonoccurrence, because they could appear in the interval between observations.
During the research conducted in the Łagiewnicki Forest, three species new to the biota of Poland were found: Diderma saundersii, Oligonema flavidum, and Didymium eximium. Didymium eximium and Oligonema flavidum were recorded only once in the “Łagiewnicki Forest” reserve, Diderma saundersii was recorded seven times in the reserve and outside it in the Łagiewnicki Forest. Macromorphological and micromorphological specimens of these taxa are typical of these species (Poulain et al., 2011). These taxa are not rare and have been noted worldwide (Lado et al., 2013; Liu & Chang, 2011; Poulain et al., 2011; Takahashi & Iuchi, 2014).
Five taxa were recorded from the red list of myxomycetes that are rare in Poland, published in 2006 (Drozdowicz et al., 2006). The species Arcyria stipata, Physarum robustum, and Hemitrichia calyculata were recorded from various regions of the country after 2006 (Drozdowicz et al., 2012; Panek & Romański, 2010; Salamaga & Grzesiak, 2013) and from various regions of the world (“Discover Life,” 2021). Based on the available literature, Oligonema schweinitzii and Physarum flavicomum have not been reported in Poland since 2006 (Drozdowicz et al., 2006). Stemonitopsis amoena is also classified as a rare species. To date, this species is known from only one locality in Poland (Salamaga et al., 2016).
The Łagiewnicki Forest is a recreational area popular with the general public throughout the year. In the southwestern part of the Łagiewnicki Forest on the Bzura River, three water reservoirs called “Arturówek” were created, which gave rise to the largest recreational and leisure facility with a canoeing and rowing marina in Łódź. Some forest sections have adjacent residential estates, while footpaths, cycling paths, bridleways, and ski routes, as well as educational routes cross the forest. The impact of anthropopressure is evident, with littering and trails across the forest. Brushwood and branches are also sometimes collected by the public. Additionally, forest penetration outside established footpaths intensifies during the mushroom-picking season and invades the nature reserve. While littering does not have a direct impact on the occurrence of myxomycetes, picking brushwood and fallen branches inhibits their species diversity by limiting available substrates.
Despite the evident effects of anthropopressure, investigations into myxomycetes in the Łagiewnicki Forest have yielded many important findings. Based on these studies, research into myxomycetes should be conducted not only in protected areas but also in urban forest complexes.
. Summary
Investigations conducted in 2010–2012 were the first studies of myxomycetes in the Łagiewnicki Forest to record 96 taxa (91 species, five varieties), comprising 35% of the taxa known in Poland. The localities of the species Diderma saundersii, Didymium eximium, and Oligonema flavidum were reported in Poland for the first time. Five taxa – Arcyria stipata, Hemitrichia calyculata, Oligonema schweinitzii, Physarum flavicomum, and Physarum robustum – should be considered as relatively rare species in Poland. Stemonitopsis amoena should be considered rare, because it has only been reported from one location in Poland.
Observations conducted on logs of four tree species helped to gather data on species inhabiting specific ecosystems and to note succession, phenology, and co-occurrence of taxa. The greatest number of the same species occurred on Betula pendula and Quercus sp., and the smallest number of the same species occurred on Quercus sp. and Picea abies. Species diversity was lower in the second year of the observations.
Most taxa colonized a wide range of substrates. No preference for tree species was noted in myxomycetes colonizing dead wood.
The greatest number of taxa and specimens of myxomycetes were recorded on dead wood forming lying logs.
The amount of dead wood was considerably higher in the reserve than in the neighboring forest. The richness and frequency of the slime mold species were also higher. It seems that the amount of large-sized woody debris left to decay naturally is a crucial factor for myxomycete diversity.
Over 50% of the taxa recorded in the study did not exhibit a preference for a specific time period during the vegetative season.
The Łagiewnicki Forest complex lies within a city and is a recreational amenity for local residents. Urbanization and anthropopressure influence the availability of potential microhabitats. Despite this, the results of the study show that the forest is a natural site of high value, with a great species diversity of myxomycetes, where species new to and rare in Polish biota were recorded.