. Introduction
Laricifomes officinalis (Batsch) Kotl. & Pouzar is a holarctic wood-decay fungal species reported in Europe, the Urals, Siberia, Northeast Asia (China, Japan, Korea), North America, and the Atlas Mountains (North Africa) (The Global Fungal Red List Initiative, n.d.; Hayova et al., 2020; Mukhin et al., 2005; Piętka & Szczepkowski, 2004; Ryvarden & Melo, 2017; Wojewoda, 2010).
Both morphological and molecular analyses support the separation of L. officinalis from the genus Fomitopsis P. Karst. (Bernicchia & Gorjón, 2020; Binder et al., 2013; Han et al., 2016; Rivoire, 2020; Ryvarden & Melo, 2017). Nevertheless, current fungal taxonomic databases such as Faces of Fungi (https://www.facesoffungi.org/), Index Fungorum (http://www.indexfungorum.org/), and MycoBank (https://www.mycobank.org/) do not provide a congruent interpretation.
Mostly located in mountain environments (Körner et al., 2011), L. officinalis grows on conifers, especially larch (Larix decidua Mill.), which is its exclusive host in the Alps. Typically, as brown-rot agent, L. officinalis is a weak and slow necrotrophic parasite that subsequently continues to degrade wood as a saprotroph, although it generally develops basidiomata on living trees. These basidiomata are perennial and can reach several decimeters in height and a few kilograms in weight if they are not disturbed or mutilated during growth; spores are released in summer (Bernicchia, 2005; Ryvarden & Melo, 2017).
Laricifomes officinalis has been exploited for herbal and medicinal purposes throughout its distribution area as well as for ritual purposes in North America (Blanchette et al., 1992). Since the first written reference of this species by Dioscorides in the first century A.D., there has been surprising convergence in the uses of L. officinalis among different (and sometimes very distant) human populations, particularly in the treatment of pulmonary diseases, including tuberculosis, pneumonia, and asthma (Grienke et al., 2014). Nowadays, this species has been scientifically demonstrated to contain molecules (such as agaricinic acid and chlorinated coumarines) with antibacterial and antiviral activity against gram-negative bacteria, Mycobacterium tuberculosis, herpes simplex virus, Poxviridae, Orthopoxvirus, and type A influenza virus in birds (H5N1) and humans (H3N2) (Girometta, 2019). At the beginning of the twentieth century, European pharmaceutical companies were very interested in exploiting the basidiomata of L. officinalis, which brought about its overharvesting by mountaineers seeking additional earnings. This practice led to the extinction of the species in the Alps (Grienke et al., 2014; Senn-Irlet, 2012). However, currently, L. officinalis basidiomata are mostly used to add flavor to spirits and sometimes for esthetic purposes; thus, the impact of harvesting them in the Alps has decreased but not entirely ceased (Gregori, 2013).
Besides harvesting, another major threat to L. officinalis is habitat loss and fragmentation due to human activities. As mentioned earlier, in the Alps, this species is associated with the larch, particularly old individuals. European larch (L. decidua) has a life-span of 600–800 years under optimal conditions, and several individuals over 1,000 years of age have been reported in Alps (Carrer & Urbinati, 2004; Dibona, 2014; Grossoni et al., 2018; Ronch et al., 2016). Unfortunately, since the relationship between age and circumference is strongly affected by climatic, environmental, and morphological factors, it is not possible to correlate host size and age without a specific dendrochronological analysis. In any case, it takes a few decades for species to recover from the depletion of such a limited trophic niche, which has had a strong impact on L. officinalis (Juutilainen et al., 2017; Motta et al., 2006).
In the last few years, L. officinalis has been included in the red lists of at least five countries (European Council for the Conservation of Fungi, 2019; Svetasheva, 2014). More recently, The Global Fungal Red List Initiative has assessed and published this species as endangered (The Global Fungal Red List Initiative, n.d.). According to Gregori (2013) and Senn-Irlet (2012), this species and its habitat have been specifically and successfully protected in Switzerland and Slovenia, respectively.
In Italy, L. officinalis has never been included in the red lists (Rossi et al., 2014) nor has it been given a specific protection status outside natural reserves where harvesting of all macrofungi is usually forbidden. Although population census and niche modeling are of major concern for the conservation of this species (Heilmann-Clausen et al., 2015; Parolo et al., 2008), the consistency of its subpopulations in Italian alpine valleys as well as its environmental and anthropogenic drivers have never been investigated.
The present research on L. officinalis had the following aims: (i) census and spatial analysis of host trees as a small-scale proxy to assess environmental preferences; (ii) barcoding of strains in pure culture and achievement of ITS sequences for further studies on variability based on molecular comparison; and (iii) measurement of growth rates and description of standardized growth profiles to support strain selection for future studies and applications.
The present study is the first step in a broad-scale investigation. The molecular diversity of L. officinalis has been poorly investigated worldwide and never in the Alps.
. Material and Methods
Study Area
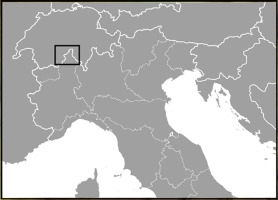
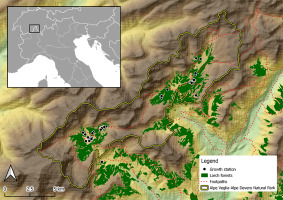
Sampling was conducted in the Parco Naturale Alpe Veglia–Alpe Devero (Alpe Veglia–Alpe Devero Natural Park) in the province of Verbano-Cusio-Ossola (VCO), Italy, as shown in Figure 1.
The park territory ranges between 1,500 and 3,552 m above sea level, mostly in the subalpine belt (approximately 1,500–2,200 m), followed by the alpine belt (approximately 2,200–3,000 m). Larch is the widely dominant tree species forming open woods, which are included in Habitat 9420 according to EU Directive 92/43/CEE (Council of the European Union, 1992). The park is therefore included in the central-western endalpic acidophilous geosigmetum of Italian vegetation (Blasi, 2010).
The park includes the western side valleys of Val d’Ossola (VCO): Valle Antrona, the Alpe Veglia basin (Val Cairasca), the Alpe Devero basin, and the Agaro basin (Val Devero). According to the hierarchical arrangement of the terrestrial Italian ecoregions, Val d’Ossola is located in the Northwestern Alps subsection, the Western Alps section, the Alpine province, and the Temperate division (Biondi et al., 2014; Blasi et al., 2014). Thus, the phytoclimate in the side valleys of Val d’Ossola is temperate oceanic, and hyperhumid orotemperate and cryotemperate ultrahyperhumid/hyperhumid at lower and higher altitudes, respectively (Ministry for Environment, Land, and Sea Protection of Italy, 2014). Alpine forests of L. decidua and/or Pinus cembra L. have consequently been recognized as priority habitats (code 9420) within protected areas (Casale & Pirocchi, 2005).
Sampling and Mapping
All basidiomata of L. officinalis were counted and labeled according to the general ground mapping and count technique described for ecological census (Sutherland, 2006). Sampling was performed in 2015, 2016, and 2018. The only stratification applied to the sampling area was because of inaccessibility due to topographical factors; as a whole, uncovered areas represent less than 1/20 of the total sampling area.
All host trees were geo-localized using a global positioning system (GPS; Midland AlanMap 500). To minimize bias due to potentially insufficient signal reception, all data were subsequently checked by exploiting the institutional territory information systems of Regione Piemonte (http://www.geoportale.piemonte.it/). In the case of aggregated samples, that is, when there is a very small distance between host trees, manual triangulation by CTR – Technical Regional Map (http://www.geoportale.piemonte.it/) and compass (Recta DS 50 Global System) was also performed.
Parameters of Host Trees and Sampling Area
The trunk circumference of host larches was measured 1 m above the soil level (upslope). The average height of the attachment point to the larch trunk was also measured for most basidiomata. Using a digital elevation map (DEM) of Regione Piemonte with a spatial resolution of 20 m, the elevation, aspect, and side inclination were measured at the host trees as well as the Terrain Ruggedness Index (TRI). TRI is a measure of the terrain asperity and was calculated as the average difference between the elevation at the host trees and the eight surrounding pixels of the DEM used. Furthermore, using the land cover map (1:25,000) (Land Cover Piemonte: Classificazione uso del suolo 2010, 2019) and the forest map of Regione Piemonte (1:25,000) (Carta forestale, 2020), the land use cover and forest type at host trees were also defined. The Alpe Veglia area was calculated as the hydrographic basin closed at point 435445 E, 5122636 N (coordinate reference system WGS84 32N, EPSG: 32632), whereas the Alpe Devero area was calculated as the hydrographic basin closed at points 443330 E and 5128359 N (http://www.geoportale.piemonte.it/geocatalogorp). In addition, the distance from the footpaths was measured using a regional footpath map (1:25,000) (Ppr – Rete sentieristica, 2020). The minimum convex polygon (also called convex hull) was calculated as the area containing the sampled host trees (Graham, 1972). Spatial analyses were carried out using QuantumGIS v.3.8.3 “Zanzibar.”
We tested whether the environmental characteristics of the growth sites in the study area differed between Alpe Veglia and Alpe Devero using the standard nonparametric method because of the small sample size (Legendre & Legendre, 1998). For continuous variables, we used the Mann–Whitney U test for two independent samples, whereas for categorical variables, we used Fisher’s exact test for the obtained data.
Isolation and Identification of Laricifomes officinalis Strains
Basidiomata were harvested whenever possible and only once per host tree. Identification of the basidiomata was confirmed by macro- and micromorphological analyses (Bernicchia, 2005; Ryvarden & Melo, 2017).
Isolation of mycelia in pure culture was performed under sterile conditions by inoculating small portions of the context into Petri dishes containing MEA medium (malt extract 2% + agar 1.5%) with the addition of chloramphenicol 50 ppm. Incubation was performed in the dark at 25 °C, and the growth of each strain was checked daily for a month.
Molecular identification of pure cultures was carried out on mycelia cultured in a liquid medium (malt extract 2%). DNA was extracted from lyophilized mycelia using a DNeasy Plant Kit (Qiagen). PCR amplification was carried out using the primer pair ITS1 (TCCGTAGGTGAACCTGCGG) and ITS4 (TCCTCCGCTTATTGATATGC) for the internal transcribed spacer (ITS) of ribosomal DNA; this region has been widely used for different fungal taxa (Hyde et al., 2013; Nilsson et al., 2012). The PCR protocol used Dream Taq Mastermix (Promega) and was performed in a thermocycler, as reported by Cesaroni et al. (2019).
Qualitative verification of DNA (5 µl/sample) was performed both after DNA extraction and PCR amplification by DNA run (30 min, 100 V) on an electrophoretic gel (1% agarose). SYBR Safe-DNA gel stain (Invitrogen) was used as an intercalant, GeneRuler 1kb (Thermo Scientific) was used as a ladder, and BlueJuice (Invitrogen) was used as a gel loading buffer. Imaging was performed using a Gel Doc (Bio-Rad).
CleanSweep PCR Purification Reagent (Applied Biosystems) was used to purify the amplified products. According to the suggested protocol, the reaction was carried out in a thermocycler in two steps: 45 min at 37 °C and 15 min at 80 °C.
Sequencing was performed in Macrogen. Sequence analysis was performed using the SEQUENCHER 5.0. The sequences were finally matched with those available in MycoBank.
Growth Curve and Growth Rate of Laricifomes officinalis Strains
The growth curve of L. officinalis strains was determined on different agar media.
All the isolated strains were grown in Petri dishes (9-cm diameter) containing the following media: MEA (malt extract 2% + agar 1.5%), cellobiose agar (cellobiose 0.86% + agar 1%), and agar water (agar 1.5%). ME and agar were purchased from Biokar Diagnostics, and cellobiose was purchased from Sigma Aldrich. Pinpoint inocula were set as far as possible from the center of the dish to provide more space for radial growth.
In addition, two Italian strains that had previously been isolated by authors from Val Malenco (MicUNIPV F.o.1; province of Sondrio – SO) and Gran Paradiso National Park (MicUNIPV F.o.2; province of Aosta – AO) were tested as intraspecific references (Savino et al., 2014).
The growth curves of all isolated strains were represented by reporting the colony radius as a function of time. The growth rate was calculated as the slope of the regression line interpolating the growth radius as a function of time.
. Results
Spatial Distribution of Host Trees and Relation With Topographical Parameters
The host trees of L. officinalis were only found in Alpe Veglia and Alpe Devero (Figure 2).
Figure 2
Detailed map of Alpe Veglia and Alpe Devero in Val d’Ossola. Black dots indicate host trees of Laricifomes officinalis.
All basidiomata grew on living L. decidua, despite the trees often showing old-date lesions and/or visible symptoms of pathology (branch drying, brown rot). Nevertheless, it was not possible to confirm that L. officinalis was the only causal agent of the latter.
Alpe Veglia had more host trees (24) than Alpe Devero (17); the population density of Alpe Veglia (number of individuals/area, i.e., id/km2), calculated versus the comprehensive hydrographic basin, was higher than that of Alpe Devero (0.6 id/km2 versus 0.3 id/km2). The minimum convex polygon was smaller in Alpe Veglia (2.96 km2) than in Alpe Devero (8.39 km2). Consistently, the average distance between host trees was smaller in Alpe Veglia than in Alpe Devero (see supplementary material).
Population density is related to a constant area defined a priori; a hydrographic basin in an alpine–subalpine range typically includes areas that are not expected to be populated by trees (e.g., higher moraines, tundra, rock walls, and glaciers). The minimum convex polygon is usually smaller than the actual area occupied by the subjects under examination (in our case, host trees). Thus, the minimum convex polygon is directly related to the clustering of individuals within a constant area. This means that individuals are more clustered in Alpe Veglia and more scattered in Alpe Devero. Moreover, this is consistent with the presence of wide habitat gaps in the core of the Alpe Devero basin, mainly the Devero Plain (about 0.6 km2 occupied by villages and meadows) and the Devero Lake (almost 1 km2).
The elevation of host trees ranged between 1,427 m and 2,002 m in Alpe Veglia and between 1,741 m and 2,022 m in Alpe Devero. The circumference of host larch trunks ranged between 2.20 m and 4.85 m in Alpe Veglia and between 2.30 m and 4.10 m in Alpe Devero (see supplementary material). Most host trees were in the range of 1,700–1,900 m in terms of elevation and 3–4 m in terms of host circumference (Figure 3, Figure 4).
Figure 3
Relation between the elevation (m above sea level) and aspect (azimuth). Note that nine of 24 host trees in Alpe Veglia do not have an aspect because they are located in Veglia Plain.
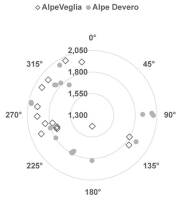
Figure 4
Relation between the host tree circumference (m) and aspect (azimuth). Note that nine of 24 host trees in Alpe Veglia do not have an aspect because they are located in Veglia Plain.
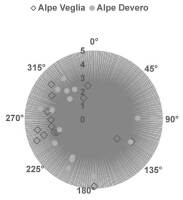
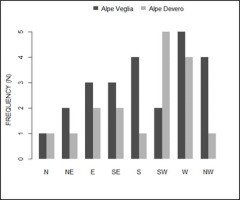
In both Alpe Veglia and Alpe Devero, host trees were mainly located on the west side; however, several were located on the plain (namely in Alpe Veglia) and it was not possible to assess their aspect (Figure 5).
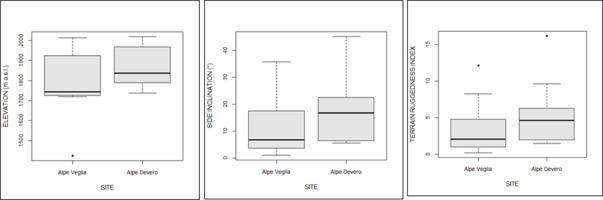
Spatial analyses showed that host trees in Alpe Veglia were at a lower elevation, along sides that were less inclined or harsh (Figure 6).
Figure 6
Comparison of the elevation (m above sea level), side inclination, and terrain ruggedness between host trees in Alpe Veglia and Alpe Devero.
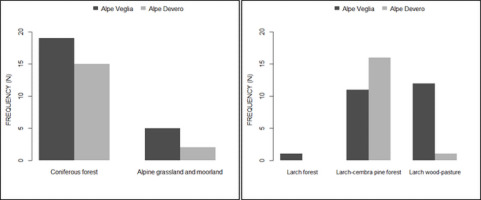
Consequently, the frequency of host trees in larch wood-pastures was higher in Alpe Veglia than in Alpe Devero, where basidiomata were mainly found in the larch-cembra pine forests (Figure 7).
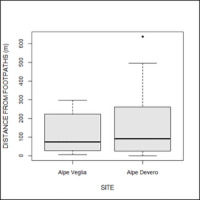
Nevertheless, no differences were found in terms of aspect, land use cover, or distance from footpaths between the two sites (Figure 8).
Figure 8
Comparison of the distances from footpaths between host trees in Alpe Veglia and Alpe Devero.
The results of spatial analyses are summarized in Table 1.
Table 1
Summary of the results of spatial analysis.
Location of Basidiomata and Morphological Features of Harvested Specimens
Basidiomata of L. officinalis did not show a preferential aspect on the tree trunk; even on the same tree, different azimuthal angles were found, indicating that colonization is generally widespread in the trunk and branches and local microenvironmental conditions (e.g., shade, wind) do not affect basidiomata development.
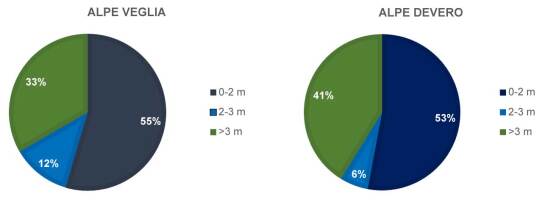
By considering the overall population of Alpe Veglia and Alpe Devero, basidiomata height above the soil level ranged between 0 and 5.5 m (average, 2.4 m). There was no significant difference between basidiomata heights in Alpe Veglia and those in Alpe Devero, as shown in Figure 9 and confirmed by an independent-samples t test (F = 1.56, α = 0.218; t = −0.397, α = 0.693).
This suggests that the colonization trend over time is similar for the two sites, because only well-established colonies can sprout basidiomata. Basidiomata show typical morphology and habitus (Bernicchia, 2005; Ryvarden & Melo, 2017). In the harvested specimens, the dry mass ranged between 0.170 kg and 0.896 kg.
Isolation of Mycelia in Pure Culture and Molecular Identification
Pure cultures of mycelia were isolated from all harvested basidiomata, comprising nine strains from Alpe Veglia and six strains from Alpe Devero. They were stored in the MicUNIPV fungal strains collection with the two Italian strains that the authors had previously isolated from Val Malenco (MicUNIPV F.o.1; province of Sondrio – SO) and Gran Paradiso National Park (MicUNIPV F.o.2; province of Aosta – AO) and are used as intraspecific references (see supplementary material). The ITS sequences from this study were used (i) to confirm the identity of strains in pure culture and (ii) to provide a certified dataset for integration with further molecular markers for subsequent studies on intra- and interspecific variability.
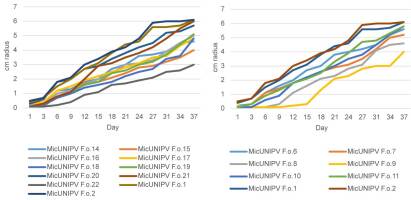
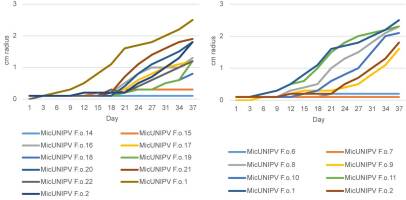
Mycelial Growth Curves
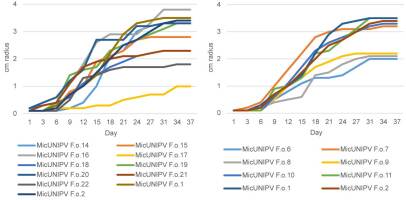
Growth curves of all isolated strains from the Alpe Veglia–Alpe Devero Natural Park are reported for different media (Figure 10–Figure 12). Growth curves of MicUNIPV F.o.1 and MicUNIPV F.o.2 are reported as intraspecific references.
Figure 11
Growth curves of strains from Alpe Veglia (left) and Alpe Devero (right) on cellobiose agar.
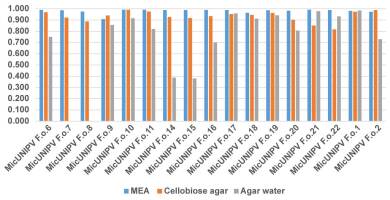
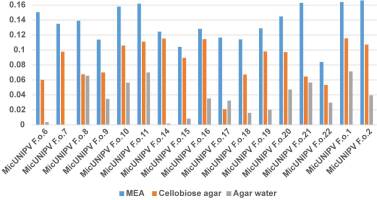
Owing to the highly significant coefficient of linear regression for all curves (Figure 13), the growth rate was considered to be approximated by the slope (Figure 14).
As expected, the highest growth rate was reported for all strains on MEA medium, whereas growth rates were lower on cellobiose agar and lowest on agar water, as it is a very poor medium. No significant correlations were found among growth rates on different media. All strains from Alpe Veglia–Alpe Devero Natural Park had lower growth rates than the control strains from Val Malenco and the Gran Paradiso National Park. Among strains from Alpe Veglia–Alpe Devero Natural Park, MicUNIPV F.o.21 (from Alpe Veglia), MicUNIPV F.o.11 (from Alpe Devero), MicUNIPV F.o.10 (from Alpe Devero), and MicUNIPV F.o.6 (from Alpe Devero) had the highest growth rates on MEA medium. Furthermore, MicUNIPV F.o.16 (from Alpe Veglia), MicUNIPV F.o.14 (from Alpe Veglia), MicUNIPV F.o.11 (from Alpe Devero), and MicUNIPV F.o.10 (from Alpe Devero) had the highest growth rates on cellobiose agar.
. Discussion
According to the literature, L. officinalis was assumed to be rare in Italy, with only sporadic reports in the past few dedades (Bernicchia, 2005; Onofri, 2005). From a methodological point of view, this census of L. officinalis basidiomata can be considered reliable, with only a few sectors remaining uncovered. Counts are generally regarded as a bias-free method for measuring the true population density of a species (Sutherland, 2006). Underestimation of the population consistency due to the inaccessibility of some areas can be considered negligible because of the small size of most of the larches in these areas.
The present study reports that L. officinalis is more common than previously expected, suggesting that the current occurrence of this species in Alpe Veglia and Alpe Devero should be investigated considering three main factors: habitat quality, conservation efforts, and proximity to areas where this species is common and widespread.
Regarding habitat quality, apart from considerations of the age structure of larches, Alpe Veglia and Alpe Devero show a shift towards more advanced age and higher trunk diameter of hosts, particularly in Alpe Veglia (Garbarino et al., 2013); this is consistent with the larger number of L. officinalis basidiomata in this area. The similar distribution of basidiomata height above the soil in both Alpe Veglia and Alpe Devero suggests that the colonization trend over time is similar for the two sites, because only well-established strains can develop basidiomata. In any case, no considerations can be made regarding the preferential height of the infection point owing to the lack of drilling transects along the trunks. Therefore, a further methodological step in future studies on L. officinalis may require the support of dendrochronology and/or molecular methods.
Regarding the proximity to areas where L. officinalis is especially common and widespread, it should be considered that the sampling areas border Switzerland (Valais). Our results are consistent with the recent increase in the Swiss population of L. officinalis, particularly close to the Italian borders in the upper Rhône Valley (Valais), where reports have become more frequent (Senn-Irlet, 2012).
Another major factor affecting sexual reproduction and consequently basidiospore spreading in L. officinalis is the harvesting of basidiomata by humans. This seems to impact the species despite the prohibition of the harvesting of any fungal species within the natural park. Basidiomata height above the soil (Figure 9) is not the only factor preventing humans from collecting them, which is consistent with the data shown in Figure 6 and Figure 8; side inclination and ruggedness contribute to inaccessibility more than distance from footpaths.
Laricifomes officinalis is known to be a very slow-growing species in pure culture (Stalpers, 1978); thus, strains with lower growth rates are particularly evident over time, because there is a remarkable difference between them and the best-performing strains. All possible applications of L. officinalis mycelia should rely on preliminary strain characterization; hence, we chose to test and compare a large number of strains.
Although MEA is a rich medium that allows fast assimilation, cellobiose agar was expected to promote higher growth rates than were observed, because L. officinalis is a typical brown-rot agent and cellobiose dehydrogenase activity is expected to be significant within a few days after inoculation (Doria et al., 2014).
By combining growth rates reported on both MEA and cellobiose agar, strains MicUNIPV F.o.11 and MicUNIPV F.o.10 from Alpe Devero seem to be the most versatile and fast-growing. Strains from the Alpe Veglia–Alpe Devero Natural Park were isolated more recently (2015, 2016, and 2018) than the control strains from Val Malenco (2013) and Gran Paradiso National Park (2013). Nevertheless, the control strains had higher growth rates in all media, suggesting that L. officinalis strains may lower their growth rate only after several years, i.e., strains stored for few years (5 years in this study) do not lower their growth rate. Growth rate is not the only parameter to evaluate physiological functionality, and it is possible that these isolates characterize strains with different physiological properties. However, the growth rate is a fundamental criterion for selecting strains, as physiological tests require sufficient biomass to be produced in a reasonably short time by actively growing colonies.
. Conclusions
The basidiomata census in the Val d’Ossola (VCO, Italy) confirms that L. officinalis is closely associated with the presence of significant populations of old, living larches (L. decidua). Legal protection has prevented basidiomata of L. officinalis from being over-harvested, which was the main factor underlying the dramatic decline of this species in the twentieth century.
Based on the findings discussed above, L. officinalis appears to be a resilient species, at least in areas where a sink source has settled or in neighboring areas, as long as its habitat is preserved and the species has protected status, not only within, but also outside natural reserves. Since old living larches are essential substrates for this fungal species, forest management should plan small forest/tree reserves throughout the subalpine belt of the Alps to ensure the stability of the population of this medicinal wood decay polypore. In cases of limited resources or a lack of effective solutions, single larch individuals or small clusters may be protected as microreserves (Kadis et al., 2013).
In conclusion, the legal protection of L. officinalis and conservation of its habitat cannot be separated. Legal protection of this species in the Italian Alps is a fundamental tool to add value to this Alpine resource, even though L. officinalis has not been reported in the Italian red lists. The Italian red lists of fungi are still in the initial stages of preparation, with the current version only including a few species that are all regarded as “nonpolicy species” (Rossi et al., 2013), and these red lists follow the International Union for Conservation of Nature (IUCN) protocol, similar to the equivalent red lists for vascular flora (International Union for Conservation of Nature, 2012). On a national scale, L. officinalis is a difficult case to manage because the population trend of the fungus is apparently positive (criterion A) and larch forests are neither threatened nor rare habitats (criterion B).
The case of L. officinalis shows that the dichotomy between national and regional scales should be carefully evaluated because the population may only be abundant on a local scale. Furthermore, the decision by The Global Fungal Red List Initiative to assess and publish this species as “endangered” (Dahlberg & Mueller, 2011) is based on the awareness that the protection policy must prevent a species from having or returning to a critical status even when its population trend is apparently positive.
. Supplementary Material
The following supplementary material is available for this article:
Table S1: Euclidean natural distances between the host trees.
Table S2: Main parameters of host trees.
Table S3: Summary of the strains isolated from Alpe Veglia–Alpe Devero Natural Park with respect to the comprehensive pool of samples.