Introduction
For several dozen years, the phenomenon of the disappearance of Cladonio-Pinetum communities and their transformation into more eutrophic forest communities has been observed (Danilewicz & Pawlaczyk,2004; Prieditis,2002; Węgrzyn & Wietrzyk,2017; Zaniewski et al.,2012,2015). In the “Bory Tucholskie” National Park (PNBT), preserving the natural habitat of the Central European lichen Scots pine forests (91T0) with an area of 930.58 ha (Matuszkiewicz et al.,2012; Solon & Matuszkiewicz,2012) is one of the priority protection objectives (Minister of Environment,2008). Therefore, in 2015, expert opinions were sought to explain the disappearance of this forest type in large forest complexes by comparing well-preserved forest patches with those representing an intermediate and degraded form. Results of this inquiry were used to propose active protection procedures (Węgrzyn & Wietrzyk,2017); in addition, lichenological and mycological inventories were made (Grzesiak, Kochanowska, & Kochanowski,2017; Węgrzyn & Wietrzyk,2017). The research of macrofungi was carried out using both the route method and permanent plots representing the most typical fragments of the community. In total, 140 taxa of macroscopic fungi were identified (76 in 2014, 90 in 2015, and 98 in 2016) (Grzesiak, Kochanowska, & Kochanowski,2017), which is 28% of the mycobiota in PNBT (517 taxa) (Grzesiak, Wolski, et al.,2017; Ławrynowicz,2012). Such expertise and efforts led to the active protection measures taken in 2017–2018 to maintain these valuable habitats and prevent their disappearance. The patches of the community were selected in which the processes of plant succession are visible, contributing to the reduction of the natural habitat (total area of 12.63 ha). The aim of the management activities was to reduce the factors leading to the loss of the community, primarily shading and eutrophication (Dingová-Košuthová et al.,2013; Węgrzyn & Wietrzyk,2017; Zaniewski et al.,2015). Examples of these activities are (i) regulation of the density of forest stands in order to reduce the forest cover to the optimal level for Cladonio-Pinetum forests (i.e., 0.6–0.7, on an area of 12.63 ha), (ii) removal of wood from the surface after loosening cut and dead wood on the forest floor (over 12.63 ha), (iii) raking forest litter in an area of 3.43 ha, and (iv) removing bryophytes in an experimental area of 0.42 ha.
The aim of the mycological study was to create an inventory of macroscopic fungi in the Cladonio-Pinetum communities in the area covered by these active protection treatments.
Material and Methods
Materials included macromycetes collected within and outside of the research plots, in the area occupied by Cladonio-Pinetum forest sections. In December 2017, 24 permanent circular plots, each with an area of approximately 100 m2, were established in Forest Sections 18 and 19 (Forest Subsections: 18c and 19d, g, h, i, j, and k) (Figure 1), which have been marked permanently in the field. The geographical coordinates of the center of each plot, determined by a Garmin Colorado 300 GPS receiver, were recorded and applied to the base GIS PNBT.
Figure 1
(A) Location of permanent plots (blue, numbered 1–24) and loggers (red). The numbers and small letters (purple) indicate forest sections and forest subsections. Areas in green are areas of Cladonio-Pinetum designated by experts for active protection procedures. (B) Location of forest sections in the area of “Bory Tucholskie” National Park. Map in (A) was prepared by Agnieszka Turowska; map in (B) is modified from Grzesiak, Kochanowska, and Kochanowski (2017).
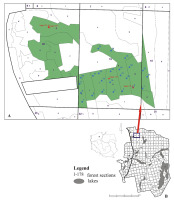
Permanent Plots 1, 8, 9, and 22 were located on a slight elevation, and the others were located on a flat or slightly lower area. Lichens and bryophytes were found in the undergrowth in all of the plots. Twigs, stumps, cones, and needles were found on all surfaces, constituting the substrate for saprotrophic fungi. Birches (Betula pendula) were present in the vicinity or within Plots 4, 5, 11, 12, 13, and 22. In September 2018, a litter removal procedure was carried out on Plots 3, 7 (in part of the area), 8, 10, 16, and 19 (on the whole area), and, consequently, mineral soil was exposed.
Mycological observations were carried out once a month, from April to November 2018. The material was identified as much as possible in the field in a fresh state and, in the case of questionable species, through collected specimens of fungi. Sporocarps of protected species of fungi were not collected. In the case of protected fungi and endangered and rare species, GPS coordinates were noted. Photographic documentation was carried out by means of a Canon EOS 750D.
Collected materials were studied with standard methods used in the taxonomy of macromycetes. Microscopic characteristics were viewed using a Nikon Eclipse 50i light microscope and an Olympus SZ61 stereoscopic microscope (binocular). Specimens were identified by examining macroscopic and microscopic features, using monographs by Antonín and Noordeloos (2010), Bas et al. (1988,1990,1995,1999), Breitenbach and Kränzlin (1984,1986,1991,1995,2000), Galli (1996), Knudsen and Vesterholt (2012), and Kränzlin (2005).
In this study, fungi were divided into several ecological groups: (i) terricolous fungi growing on soil, humus, sand; (ii) litter-inhabiting fungi colonizing the litter in the form of needles, small fallen twigs, leaves, grass blades, and dead herbs; (iii) xylobiontic fungi occurring on wood (logs, tree trunks, stumps, roots, branches); (iv) bryophilous fungi colonizing mosses; (v) cone-inhabiting fungi growing on cones; and (vi) fungicolous fungi occurring on old sporocarps of other fungi. They were categorized into these groups based on the substrate colonized [according, for instance, to Knudsen and Vesterholt (2012)]. Three categories of fungi have been distinguished based on the living form of individual species: (i) mycorrhizal fungi, (ii) saprotrophic fungi, and (iii) parasitic fungi [also according to Knudsen and Vesterholt (2012), and the monographs mentioned above].
The nomenclature of basidiomycetous fungi was accepted after Knudsen and Vesterholt (2012). The nomenclature of species not included in Kundsen and Vesterholt (2012) and species of ascomycetous fungi are in accordance with the Index Fungorum (2019). Protected species are those listed by the regulation of the Minister of the Environment on the protection of fungal species (Minister of Environment,2014), with threat categories according to Wojewoda and Ławrynowicz (2006).
Results: List of Macromycetes
Abbreviations and indications: 1–24 – numbers of permanent plots; Roman numerals – months of note taxon; f.s. – forest section; * – fungi recorded beyond permanent plots; RL – “Red list of the macrofungi in Poland” (Wojewoda & Ławrynowicz,2006) categories: E – endangered, V – vulnerable, R – rare, I – indeterminate.
Amanita excelsa (Fr.: Fr.) Bertill. f. excelsa [= A. spissa (Fr.) O. Kumm.] – on soil, among mosses and lichens, under pines; 1, IX.
Amanita gemmata (Fr.) Bertill. – on soil, among lichens and mosses, under pines; 5, IX.
Amanita muscaria (L.: Fr.) Pers. var. muscaria – on soil, among mosses, under pines and birches; 4, IX–XI; 14, 15, X–XI; 12, 13, 20, X; 3, 8, 11, XI.
Armillaria borealis Marxm. & Korhonen – on stumps and deadwood of birch; 11, XI.
Ascocoryne sarcoides (Jacq.) J. W. Groves & D. E. Wilson – on stump of birch; 11, XI.
*Boletopsis grisea (Peck) Bondartsev & Singer – on soil, among mosses and lichens, under pines; f.s. 19d, X–XI. Protected species. RL – E.
Boletus edulis Bull.: Fr. – on soil, among lichens, under pines; 16, XI.
*Cantharellus cibarius Fr. – on soil, among mosses, under pines; f.s. 19f, l, VIII.
Clitocybe metachroa (Fr.: Fr.) P. Kumm. var. metachroa – on coniferous litter, among mosses; 21, X.
Clitopilus prunulus (Scop.: Fr.) P. Kumm. – on soil, among mosses; 7, XI.
Collybia cirrata (Schumach.) Quél. – on decaying sporocarps of other fungi, among mosses; 3, X.
Collybia tuberosa (Bull.: Fr.) P. Kumm. – on decaying sporocarps of other fungi; 1, X.
Coltricia perennis (L.) Murrill – on soil; 14, VI–IX; 6, IX.
*Cortinarius armeniacus (Schaeff.: Fr.) Fr. – on soil, among mosses and lichens, under pines; f.s. 18d, IX. RL – V.
Cortinarius brunneus (Pers.: Fr.) Fr. – on soil, among lichens; 1, XI.
Cortinarius caperatus (Pers.: Fr.) [= Rozites caperatus (Pers.: Fr.) P. Karst.] – on soil, among mosses and lichens, under pines; 1, IX; 6, IX, XI; 21, XI.
Cortinarius cinnamomemus (L.: Fr.) Gray – on soil, among lichens; 3, XI.
Cortinarius mucosus (Bull.: Fr.) J. J. Kickx – on soil, among mosses and lichens, under pines; 4, IX, XI; 23, X; 1, 11, 14, 17, XI.
Cortinarius semisanguineus (Fr.: Fr.) Fr. – on soil, among mosses, under pines; 1, XI.
Cortinarius obtusus (Fr.: Fr.) Fr. s. lato – on soil, among mosses, lichens and heathers, under pines; 3, XI.
Cystoderma amianthinum (Scop.: Fr.) Fayod – on soil, among mosses; 1, 20, 21, XI.
Dacrymyces capitatus Schwein. – on small fallen branches of pine; 2, VI; 12, X. RL – V.
Dacrymyces stillatus Nees – on small fallen branches of pine; 12, X.
Deconica montana (Pers.: Fr.) P. Kumm. – on mosses (Polytrichum piliferum); 22, XI. RL – R.
Entoloma rhodocalix (Lasch: Fr.) M. M. Moser – on a well-decayed stump of pine; 16, VII. RL – R.
Galerina hypnorum (Schrank: Fr.) Kühner s. Horak 2005, de Haan & Walleyn 2006 – on mosses (Dicranum sp., Pleurozium schreberi); 1, 4, 21, X–XI; 2, 3, 5–7, 9, 11, 15, 18, 20, 22–24, XI.
Gloeophyllum sepiarium (Wulfen: Fr.) P. Karst. – on stump of pine; 14, X–XI.
Gymnopilus penetrans (Fr.) Murill – on branches of birch; 12, 14, 22, XI.
Gymnopilus sapineus (Fr.: Fr.) Maire s. Kühner & Romagnesi, Moser, Ludwig, Breitenbach & Kränzlin, Horak, non Fries 1821, Hᴓiland 1990 – on dead wood of pine; 2, 10, 19–21, 24, VII.
Gymnopus androsaceus (L.: Fr.) J. L. Mata & R. H. Petersen [= Setulipes androsaceus (L.: Fr.) Antonín] – on needles of pine; 1, VII–XI; 2, VIII–XI; 3, 6. 12, 13, 21, IX–X; 4, 11, VII, IX–XI; 5, 9, IX–XI; 7, 14–16, 18, 20, 23, X; 17, VIII, X–XI.
Gymnopus putillus (Pers.: Fr.) (Fr.: Fr.) Antonín, Halling & Noordel. – on litter; 1, 10, X; 8, 20, 23, 24, XI.
Heterobasidion annosum (Fr.) Bref. – at the base of trunks of pine; 20, VI.
*Hygrophoropsis aurantiaca (Wulfen: Fr.) Maire – on coniferous litter, among mosses; f.s. 18d, XI.
Hypholoma capnoides (Fr.: Fr.) P. Kumm. – on dead wood of pine; 13, 14, XI.
Laccaria laccata (Scop.: Fr.) Cooke – on soil, among lichens and mosses, under pines; 1, 9, 14, 15, 17, XI; 3–5, 11, 13, 22, X–XI.
Lactarius necator (Bull.: Fr.) Pers. [= L. turpis (Weinm.) Fr.] – on soil, among mosses and lichens, under birches; 4, IX–X; 11, 12, IX.
Lactarius rufus (Scop.: Fr.) Fr. – on soil, among mosses and lichens, under pines; 1, 3, 9, X; 7, 21, 22, XI.
Lactarius vietus (Fr.: Fr.) Fr. – on soil, among mosses, under birches; 4, XI.
*Leccinum scabrum (Bull.: Fr.) Gray – on soil, among lichens, under birches; f.s. 19i, IX.
Leccinum versipelle (Fr. & Hök) Snell – on soil, among mosses and lichens, under birches; 4, IX.
*Lyophyllum fumosum (Pers.: Fr.) P. D. Orton [= L. conglobatum (Vitt.) M. M. Moser, L. decastes (Fr.: Fr.) Singer var. fumosum (Pers.: Fr.) Kühner] – on soil, among mosses; f.s. 17d, IX.
Mycena cinerella (P. Karst.) P. Karst. – on coniferous litter; 4–7, 23, XI.
Mycena galopus (Pers.: Fr.) P. Kumm. – on coniferous litter, among mosses; 3, 4, VII; 11, XI.
Mycena metata (Fr.: Fr.) P. Kumm. – on coniferous litter; 4, XI.
Mycena vulgaris (Pers.: Fr.) P. Kumm. – on coniferous litter, among mosses; 12, VII; 5, 6, X; 1, XI.
Paxillus involutus (Batsch: Fr.) Fr. – on soil, among mosses, under pines; 1, 17, XI; 2–7, 10, 12, 13, 15, 18, 20, 22, 23, X–XI; 9, 21, 24, IX–XI; 11, 14, 16, X.
Pholiotina filipes (G. F. Atk.) Singer – on coniferous litter, among mosses; 4, 6, XI.
*Russula adusta (Pers.: Fr.) Fr. – on soil, among mosses and lichens, under pines; f.s. 17d, IX.
Russula betularum Hora – on soil, among mosses and lichens, under birches; 4, 22, IX.
Russula decolorans Fr. – on soil, among mosses, under pines; 1–4, 7, 9, 10, 13, 14, 17, 18, 21, 23, IX; 6, IX–X.
Russula emetica (Schaeff.: Fr.) Pers. s. lato – on soil, among mosses, under pines; 1, 2, 4, 6, IX–XI; 5, 12, 21, XI; 7, 11, 17, 20, 23, 24, X–XI; 13, 18, IX, XI; 22, IX.
Russula paludosa Britzelm. – on soil, among mosses, under pines; 5, 18, IX.
Russula risigallina (Batsch) Sacc. var. risigallina (= R. chamaleontina Fr.) – on soil, among mosses, near pines and birches; 6, IX.
*Russula vesca Fr. – on soil, among mosses, under pines; f.s. 18c, 19i, IX.
*Sarcodon squamosus (Schaeff.) Quél. – on soil, among lichens and mosses, under pines; f.s. 19d, i, XI.
Schizophyllum commune Fr.: Fr. – on stump of pine; 2, 4, 22, X; 5, 10, IX; 14, X–XI.
Strobilurus stephanocystis (Hora) Singer – on cones of pine; 4, IX; 5, IV, IX; 8, IV.
Strobilurus tenacellus (Pers.: Fr.) Singer – on cones of pine; 1, 2, 6, 7, 17, 20, 22, 23, XI.
Suillus bovinus (L.: Fr.) Roussel – on soil, among mosses and lichens, under pines; 11, X.
Tapinella atrotomentosa (Batsch: Fr.) Šutara – near the neck of pine root; 1, 10, X.
Thelephora terrestris Ehrh. – on soil; 3, VII; 22, VIII; 24, XI.
Trichaptum abietinum (Pers.) Ryvarden – on trunks of pines; 16, VII; 8, VIII.
Tricholoma albobrunneum (Pers.: Fr.) P. Kumm. – on soil, among lichens, under pines; 9, 11, 14, 15, 17, XI.
Tricholoma colossus (Fr.) Quél. [= Megatricholoma c. (Fr.) G. Kost] – on soil, in the sand, under pines; 8, X. RL – E.
Tricholoma equestre (L.: Fr.) P. Kumm. [= T. flavovirens (Pers.: Fr.) S. Lundell] – on soil, in the sand, under pines, among lichens; 3, 22, X–XI; 7, 16, 17, XI; 20, X. RL – I.
Tricholoma portentosum (Fr.: Fr.) Quél. – on soil, among mosses and lichens, under pines; 23, 24, XI.
Tricholoma saponaceum (Fr.) P. Kumm. var. saponaceum – on soil, among lichens, under pines; 3, XI.
Tricholoma terreum (Schaeff.) P. Kumm. – on soil, in the sand, under pines, among lichens; 13, X; 14, XI.
Tricholomopsis rutilans (Schaeff.: Fr.) Singer – at the base of trunk of pine; 2, VII; 21, X.
Xerocomus badius (Fr.: Fr.) E.-J. Gilbert – on soil, among mosses and lichens, under pines; 1, 12, 14, 15, 17, X–XI; 2, 23 X; 6, 19, IX; 20, XI; 13, 21, 24, IX–XI.
Xeromphalina cornui (Quél.) J. Favre – on coniferous litter, among mosses; 1, XI.
Discussion
As a result of the observations that were carried out in the Cladonio-Pinetum community in in 2018, a total of 71 taxa of macroscopic fungi was identified (62 on permanent plots, and nine outside of them). One of the fungi, Ascocoryne sarcoides, belongs to Ascomycota, the others to Basidiomycota.
Some of the taxa found in this study were listed for the first time in the Cladonio-Pinetum community in the PNBT (Grzesiak, Kochanowska, & Kochanowski,2017). In total, there were 18 taxa: Armillaria borealis, Ascocoryne sarcoides, Boletopsis grisea, Clitopilus prunulus, Entoloma rhodocalix, Gloeophyllum sepiarium, Gymnopilus sapineus, Gymnopus putillus, Heterobasidion annosum, Lactarius vietus, Mycena cinerella, M. metata, M. vulgaris, Pholiotina filipes, Russula betularum, Schizophyllum commune, Tricholoma colossus, and T. saponaceum.
The plots with the greatest number of taxa were Plots 1 and 4 (with 20 and 18 taxa, respectively); the plot with the least number of taxa was Plot 19 (where only two taxa were recorded) (Table 1). The greatest number of fungal taxa (39) were recorded in November, while in May no sporocarps were found.
Table 1
Number of fungal taxa identified within the individual permanent plots.
| Plot | Number of fungi taxa | Plot | Number of fungi taxa |
| 1. | 20 | 13. | 9 |
| 2. | 12 | 14. | 14 |
| 3. | 14 | 15. | 7 |
| 4. | 18 | 16. | 7 |
| 5. | 11 | 17. | 10 |
| 6. | 13 | 18. | 6 |
| 7. | 10 | 19. | 2 |
| 8. | 5 | 20. | 12 |
| 9. | 7 | 21. | 12 |
| 10. | 6 | 22. | 12 |
| 11. | 13 | 23. | 11 |
| 12. | 10 | 24. | 8 |
Fungi recorded during field observations were on different substrates. The largest group included terricolous fungi (33 taxa; 54%) (e.g., Russula emetica, Lyophyllum fumosum), while fungi growing on bryophytes (Galerina hypnorum, Deconica montana), cones (Strobilurus stephanocystis, S. tenacellus), and old, decayed sporocarps (Collybia cirrata, C. tuberosa) formed the smallest ecological group (two taxa; 3% each) (Table 2).
Table 2
Number of fungal taxa in the ecological groups.
| Ecological group of fungi | Number of fungi taxa |
| Terricolous | 33 |
| Litter-inhabiting | 8 |
| Xylobiontic | 15 |
| Bryophilous | 2 |
| Cone-inhabiting | 2 |
| Fungicolous | 2 |
The number of ectomycorrhizal fungal species (29) was almost the same as that of saprotrophs (33). Their mycorrhizal partners were mainly pine (e.g., for Boletus edulis, Cortinarius mucosus, Lactarius rufus) and, to a small extent, birch (e.g., for Lactarius vietus, Leccinum scabrum, L. versipelle). Among the recorded species were Heterobasidion annosum and Armillaria borealis, which are very often dangerous parasites of the pine, but they were found here as saprotrophs. Parasitic fungi were not recorded.
In the study area, Boletopsis grisea, a species that is under strict legal protection (Minister of Environment,2014), was found (Figure 2). This fungus grows on acidic, nutrient-poor soils, among lichens and ericaceous plants. The species is known from the PNBT area from one site in Forest Section 157 in 2009 (Piotr Chybowski, data confirmed, not published). In Forest Sections 18 and 19, seven species on the red list have been identified (Wojewoda & Ławrynowicz,2006), whose preservation and protection are related to the preservation of the Cladonio-Pinetum community habitats. It is noteworthy that the species Tricholoma colossus (Figure 3) is found on the red list in category E, i.e., endangered.
Conclusion
The present survey provides new data on fungi of the Cladonio-Pinetum community and is the basis for observing the changes that will take place in this community after active protection. The list of fungi from these communities in the PNBT has grown by 18 species not yet recorded. Further observations are needed because a drought lasting almost the entire vegetation season led to unfavorable conditions for fungal sporocarps formation. Longer observations can lead to many interesting conclusions about fungi that have become specialized to live in such oligotrophic conditions, as well as about protected, rare, and endangered fungi.
Handling Editor
Anna Kujawa; Institute for Agricultural and Forest Environment, Polish Academy of Sciences, Poland; https://orcid.org/0000-0001-9346-2674




