. Introduction
The study of adaptation mechanisms and resistance of aboriginal plants, in particular Deschampsia antarctica É. Desv. Poaceae (Antarctic hairgrass), to the harsh climatic conditions of Antarctic is important for the comprehensive characterization of Antarctic terrestrial ecosystems and biota in general and will open the way for new applied research. Such an in situ study (in the natural conditions of Antarctic) is complicated because of the complexity of stress factors, such as wind speed, temperature, lighting, humidity, soil composition, and others, which can vary considerably. The creation of a collection of plants from different habitats of the Antarctic by cultivating and cloning in vitro, and growing in controlled indoor conditions could solve this problem.
The species that form the genus Deschampsia are metallophytes that can accumulate a significant amount of metals in their roots or aboveground parts. Such species can be used in the reclamation of areas that have been contaminated by zinc and lead, or degraded by mining. The usage of metalophytes on degraded areas enables the creation of sustainable and stable ecosystems, and reduces the costs of reclamation (Hanczaruk & Kompała-Bąba, 2019).
Since 2008, a collection of plants of D. antarctica collected from various habitats of the maritime Antarctic has been cultured in vitro and indoors. The plants originated primarily from the vicinity of the Ukrainian Antarctic Akademik Vernadsky Station. Several comprehensive studies of these plants have been reported (Navrotska et al., 2017, 2018; Nuzhyna et al., 2019; Spiridonova et al., 2016). Other researchers have also studied D. antarctica from different growth habitats (Androsiuk et al., 2021; Giełwanowska, 2005; Giełwanowska et al., 2005; Romero et al., 1999). One of the important practical aspects of D. antarctica is the presence of substances that protect the plant from ultraviolet light in the harsh ultraviolet radiation conditions that exist in Antarctic. These substances have antineoplastic activity (Gidekel et al., 2011; Ozheredova et al., 2015). Deschampsia antarctica is expected to be rich in molecules with anticancer activity (Silva et al., 2020). For example, a tricin derivative form D. antarctica inhibits the growth of colorectal cancer and liver metastasis (Malvicini et al., 2018). This usefulness is another important reason for studying these plants in vitro.
The aim of this study was to compare the anatomical structure of D. antarctica leaves grown in vitro from seeds collected from different habitats of the maritime Antarctic. The plants have been grown in culture in vitro for more than 7 years. These results are important for an understanding of the fixation opportunities of adaptive mechanisms of D. antarctica resistance to Antarctic stress conditions at the anatomical level.
. Material and Methods
We studied cloned in vitro plants of nine genotypes of D. antarctica. They were obtained from seeds collected from different localities of the maritime Antarctic. The localities featured distinctive growth conditions of the original mother plants (Table 1).
Table 1
Ecological conditions of localities where Deschampsia antarctica seeds were collected.
| Genotype | Origin andcoordinates | Ecological characteristics of the localities |
|---|---|---|
| G/D12-2a | Galindez Island:−65.247417°−64.252600° | Stella Point, coastal gull rock – Gull Tower, combination of open habitat and places protected by stones. Gravel and limpet shells deposits. Six m a.s.l. Northern exposition. Three m to kelp gull nest. South-polar skua nest located further south. No penguin organic influence. No human impact. TVC* – 5%–20%; Deschampsia antarctica – 1%–10%; bryophytes – 4%–10%. |
| W1 | Winter Island:−65.247517°−64.258033° | Northern coast. Rock terraces of northern-western exposition. Kelp gull biotope with limpets deposits. Nine m a.s.l.; 6 m to coastline; 6 m to kelp gull nest, more far to south-polar skua nests. No penguin organic influence. No human impact. TVC – 1%–5%; Deschampsia antarctica – <1%; bryophytes – 1%–5%. |
| L59 | Lahille Island: −65.553641°−64.394930° | Rock terrace on the southern coast. Rock slope of northern exposition on southern coast of southern cove. Ten m a.s.l.; 40 m to coastline. No skua or kelp gull nests. No penguin organic influence. No human impact. TVC – 5%–30%, Deschampsia antarctica – 1%; Colobanthus quitensis – <1%; bryophytes – 4%–25%. |
| R35 | Rasmussen Point:−65.246983°−64.085933° | Culmination of a rocky plateau in the area of the memorial cross. Northern exposition. Sixty m a.s.l.; 20 m to coastline. No skua or kelp gull nests. No registered penguin organic influence in time of seeds collection. Limited human impact. TVC – 5%–90%; Deschampsia antarctica – 1%–80%; bryophytes – 4%–89%. |
| S22 | Skua Island: −65.254933°−64.274017° | Rock slope of Finger Point, northern-western exposition. Six m a.s.l.; 10 m to coastline. Nearly 60 m to south-polar skua nest. No kelp gull nests. No penguin organic influence. No human impact. TVC – 1%–10%; Deschampsia antarctica – 0.5%–1%; Colobanthus quitensis – <1%; bryophytes – 0.5%–9%. |
| Y62Y66Y67 | Great YalourIsland:−65.233983°−64.162683° | Rock slope on the northern head. Northern exposition. Fifteen m a.s.l.; 10 m to coastline. No skua or kelp gull nests. No penguin organic influence. No human impact. TVC – 5%–50%; Deschampsia antarctica – 1%–10%; bryophytes – 4%–40%. |
| DAR12 | Darboux Island:−65.395117°−64.215083° | Northeast point in the vicinity of a rocky grotto, rocky terrace with limpet deposits. Eighteen m a.s.l.; 100 m to coastal line. Northern exposition. No kelp gull and skua nests and penguin organic influence. No human impact. TVC – 1%–5%; Deschampsia antarctica – 1%; Colobanthus quitensis – 0.3%; bryophytes – 2%–4%. |
Deschampsia antarctica plants have a 2n = 26 chromosome set. Among the groups of plants grown in vitro that were investigated for this study, there are, in addition to diploids, groups with different chromosome sets. These include plants of the DAR12 genotype [part of the root meristem cells contains B-chromosomes (2n = 26 + 0–3B)]; plants of genotype Y66, which are myxoploids (2n = 26, 36, 37, 38, 39) with rearranged chromosome morphology and triploid modal class; myxoploid plants of genotype Y67, in the root meristem of which separate cells are 2n = 13, 26, 38, but another are with a diploid modal class. The plants also differed in other parameters, including type of growth, amount of DNA, and other aspects (Navrotska et al., 2018). In particular, plants from Lahille Island grow as creepers, while plants from other localities characterized by shrub-like shape. These plants have retained their basic cytogenetic and molecular genetic parameters in the process of in vitro cultivation for 7–10 years (Spiridonova et al., 2016).
The plants were grown in vitro on Gamborg’s B-5 medium in a room with a controlled temperature of 17–19 °C, lighting of 6,500 lux, 16-hr light/8-hr dark regime, and humidity of 65%–75%. Under such conditions, each plant formed a bush over 40–60 days. The bush was divided into individual plants, which were transplanted to fresh medium. The plants were studied after 70–75 subcultures (cycles of clonal propagation in vitro).
The middle parts of the mature leaf blade of plants aged 3–4 months were used for anatomical studies. The samples were fixed with formalin–acetic acid–alcohol and poured over with gelatin using a standard procedure (Romeis, 1948). Sections 10 µm in thickness were created on an OMT-28-02E freezing microtome (KB-Technom; Ekaterinburg, Russia). The slices were stained with safranin. In addition, maceration of the leaves was conducted to study the structures of the adaxial and abaxial epidermis. Microscopic measurements were performed using a BX 41 microscope (Olympus; Tokyo, Japan) and ImageJ software (NIH; Bethesda, MD, USA). The area of the leaf epidermal cells was measured between veins, taking into account only the main elongated cells.
The Prism Graphpad 6 program (GraphPad; La Jolla, CA, USA) was used for statistical data processing. The values for different groups were compared by analysis of variance followed by Tukey’s multiple comparison test. The photos were taken with a C5050 Zoom digital camera (Olympus).
. Results
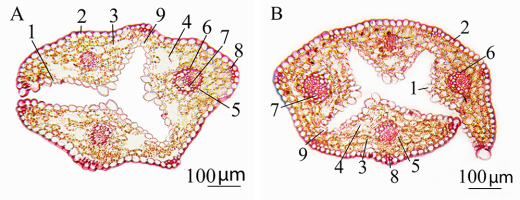
Deschampsia antarctica grown in vitro had amphistomatic leaves (Figure 1). The leaf was covered with an epidermal layer on both the adaxial and abaxial sides. The mesophyll was spongy. Large motor aquiferous cells of the epidermis were located between the ribs. The cells were the site of inward folding of the leaf blade by the adaxial side (Figure 2A,B). The vascular system was represented mainly by three vascular bundles located in the central part of each rib.
The in vitro representatives of some genotypes had distinct leaf structural features. In particular, single-celled nonglandular pointy trichomes were found on the adaxial side over the veins of genotype W1 plants originating from Winter Island (Figure 1A). Plants of genotype S22 originating from Skua Island were represented by both symmetrical three-rib and asymmetric four-rib leaves (Figure 2B). There are also five-rib leaves in plants of genotype L59 originating from Lahille Island.
Figure 1
Leaf epidermis of the Deschampsia antarctica genotype W1: (A) adaxial side (1 – simple trichoma) and (B) abaxial side.
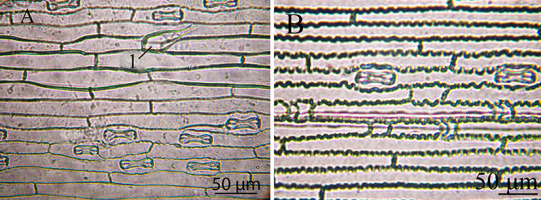
Figure 2
Cross section of the leaf of Deschampsia antarctica in vitro: (A) genotype W1 and (B) genotype S22. 1 – adaxial epidermis; 2 – abaxial epidermis; 3 – mesophyll; 4 – intercellular space; 5 – external sheath of the vascular bundle; 6 – internal bundle sheath; 7 – metaxylem; 8 – sclerenchyma; 9 – motor cells.
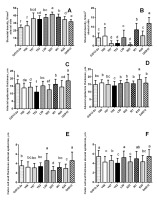
We also studied the morphometric parameters of the leaves (Table 2, Table 3, Figure 3). Genotype S22 plants had the largest number of stomata on the adaxial side (Figure 3A) and the smallest number of stomata, along with genotype Y62 and Y67 plants, on the abaxial (bottom) side (Figure 3B). The highest number of stomata on the abaxial sides of the leaves were found in genotype DAR12 (Figure 3B). Plants of genotypes Y67, S22, DAR12, and W1 had smaller stomata on both sides of the leaf and smaller epidermocytes on the bottom side, compared with the other studied genotypes (Table 2, Table 3). As the number of stomata per unit area of the leaf increased, their size tended to decrease.
Table 2
Morphometric parameters of Deschampsia antarctica genotypes in vitro (mean ± standard deviation).
Table 3
Morphometric parameters of Deschampsia antarctica genotypes leaves grown in vitro.
The most thickened outer cell walls of the epidermis were in plants of genotypes DAR12, L59, and G/D12-2a (Figure 3E,F). The thickness of the adaxial epidermis was the greatest in DAR12 plants (Figure 3C). The thickness of the abaxial epidermis was greatest in R35 plants (Figure 3D). Plants of genotypes Y62 and S22 had a relatively thin epidermis (Figure 3C,D) and outer cell walls on both sides of the leaf (Figure 3E,F). Plants of genotype L59 had the thickest and the largest of cross-section leaf blade (Table 2, Table 3).
Figure 3
Morphometric parameters of the epidermis of leaves of Deschampsia antarctica genotypes grown in vitro. The same letters indicate no significant difference between the groups (p < 0.05).
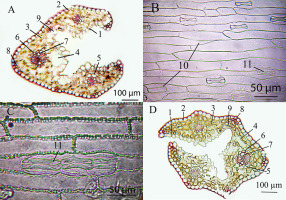
The existence of huge motor cells and the disturbance of epidermal cells (Figure 4A,B) were of note among the known anatomical differences of DAR12 plants. Also, relatively narrow vessels were found in plants of in vitro genotypes DAR12, L59, Y62, and Y67. Mesophyll cells were slightly larger in plants of genotypes R35, G/D12-2a, and W1.
Figure 4
Microphotographs of leaves of different chromosomal forms of Deschampsia antarctica in vitro. (A) Cross section of a leaf of genotype DAR12, which has the longest leaves and contains B-chromosomes. (B) Adaxial epidermis of the leaf of genotype DAR12. (C) Leaf abaxial epidermis of diploid genotype L59, which has a creeping type of growth. (D) Cross section of a leaf of genotype Y66, which is a myxoploid with a triploid modal class and rearranged chromosomes. 1 – adaxial epidermis; 2 – abaxial epidermis; 3 – mesophyll; 4 – intercellular space; 5 – external sheath of the vascular bundle; 6 – internal bundle sheath; 7 – metaxylem; 8 – sclerenchyma; 9 – motor cells; 10 – disordered epidermis; 11 – stomata.
. Discussion
In vitro grown plants have a similar anatomical structure to plants that grow in nature. We previously compared D. antarctica plants grown naturally and in vitro, and determined that anatomical differences of plants from various natural localities coincided with plants grown in vitro that originated from the same places (Nuzhyna et al., 2019). Thus, qualitative and some quantitative anatomical features are preserved upon transferring plants to in vitro culture. For example, four-rib leaves and single-celled nonglandular pointy trichomes have been previously found in plants from the natural population of the largest of the Berthelot Islands – BE1. This characteristic is completely retained in the plants in vitro (Nuzhyna et al., 2019). According to our previous research and literature data, the density of stomata in leaves of plants growing in the Antarctic is higher than in plants cultivated in the laboratory (Nuzhyna et al., 2019; Romero et al., 1999). However, the difference between the densities of stomata of different genotypes in plants propagated in vitro corresponds to that of natural plants taken from the same localities (Nuzhyna et al., 2019). The differences are especially clear on the abaxial side of the leaf in the plants being studied. The greatest density of stomata being present on the abaxial side of the leaf of the DAR12 genotype may indicate the adaptation of these plants to the arid conditions caused by low temperatures in nature, since smaller and denser stomata can be more effective at reducing water loss (Beerling & Kelly, 1996; Larcher, 1995).
Thickening of the outer cell wall of the leaf epidermis protects against dehydration, and is observed in different species of plants which are exposed to intense insolation and in regions with low water availability (Kalashnyk et al., 2016; Lobo et al., 2013; Nuzhyna & Tkachuk, 2019; Nuzhyna et al., 2020; Schreiber et al., 2006). Such thickening of the cell wall was found in plants of genotypes DAR12 and L59. We assume that epigenetic conditionality caused differences in this trait, which may have formed under the influence of aggressive conditions of the natural environment on the Darboux and Lahille islands (the southernmost, which is open from ocean side, and isolated points of the study; Table 1). In an example involving another plant species, B-chromosomes, which are present in plants of the DAR12 genotype, are more common in extreme conditions, and their presence may contribute to the adaptation of plants to such conditions (Kunakh, 2010).
Plants of genotype Y67, which had the shortest leaves and in which haploid and hypotriploid karyotypes existed in the root meristem (the plants are myxoploid with a modal diploid class) had smaller stomata on both leaf sides and smaller epidermocytes on the underside of the leaf. The abaxial epidermis of some leaves did not contain stomata, and single stomata were observed on other leaves (Table 2, Table 3). Such stomatal location features were characteristic of diploid plants of genotypes Y62 and S22 (Figure 3B). These features of the leaf structure may be due to the stability inherent to in vitro growth conditions. The stomatal density is reportedly higher in the leaves of plants growing in Antarctica than in plants cultivated in the laboratory (Romero et al., 1999). As can be seen from Table 2, Table 3 and Figure 3, the length and the width of stomata, and the epidermocytes area varied within almost the same limits between groups of plants collected from different localizations.
Myxoploid plants of the genotype Y66, in the root meristem of which triploid cells with rearranged chromosome morphology predominated, did not differ significantly from other studied plants in most parameters (Figure 4D).
The distinctive features of the L59 diploid plants that grow as creepers included the presence of double and triple stomata on the adaxial side (Figure 4C), thickened outer cell wall of the leaf epidermis, similar to DAR12, and the disordered arrangement of epidermocytes with different sizes on the adaxial side: a deviation from the norm. A similar abnormal placement of epidermal cells was found in diploid plants of genotypes W1 and R35.
Plants of genotype L59 also had notably greater thickness and a cross-sectional area of leaves compared to plants of other genotypes grown in vitro (Table 2, Table 3). However, in general, we observed contradictory data concerning the thickness and the cross-sectional area of the leaf in culture in vitro, and did not observe a specific relationship between the leaf thickness with the number of stomata, the presence of stomata on both leaf sides, or the habitat of parental plants in nature. The leaf cross-sectional area is, logically, directly proportional to the number of leaf ribs. Thus, in most in vitro plants, a leaf consisted of three ribs and was symmetrical. Plants of genotype S22 had single leaves with four ribs comprising a central rib and one and two on the sides (asymmetric). Plants of genotype L59 were characterized by five-rib symmetrical leaves, with a central rib and two on each side. According to Giełwanowska et al. (2005), D. antarctica plants growing in greenhouse conditions and in the Antarctic tundra (400 m from the seacoast) have three-rib leaves. Leaves with four–five ribs are more common in plants located near the sea in a humid environment (30 m from the sea coast).
The thick cutin layer was characteristic for all studied genotypes of Antarctic hairgrass. This feature is certainly adaptive. Cutin, suberin and waxes in the outer cell wall of the epidermis limit water loss through transpiration and protect the plant from excessive sunlight (Gunning & Steer, 1996).
The mechanical tissue in all the in vitro plants is poorly represented and consists of sclerenchymal bundles of two–five fibers under the abaxial epidermis above the vascular bundles and between the ribs. The data indicate the similarity of the degree of mechanical tissue development in plants of different origins grown in vitro compared with plants taken from nature. The dependence of the number of sclerenchymal fibers on the degree of environmental humidity, including D. antarctica, has been reported (Alvarez et al., 2008; Giełwanowska et al., 2005; Metcalfe, 1960).
High salinity and humidity affect mesophyll structure (Giełwanowska et al., 2005). Irregular mesophyll cell shapes and large intercellular spaces may promote more intense gas exchange (Korner & Larcher, 1988). According to the results of our study, all the experimental groups exhibited irregularly shaped mesophyll cells, typically found on the adaxial side under the vascular bundle. Dense rows of parenchymal cells with the correct shape were arranged under the abaxial epidermis. Also, to some extent, all the plants in the present study had large intercellular spaces in the parenchyma, especially on both sides of the vascular bundles. Thus, we did not find significant qualitative differences of the mesophyll in plants of different genotypes propagated in vitro. This can be explained by the standardization of growing conditions in the laboratory. The slightly larger sizes of the mesophyll cells of genotypes R35, G/D12-2a, and W1 may indicate the less aggressive habitat conditions of parental forms of these genotypes in nature.
The vascular bundle is surrounded by two sheaths (sclerenchymal lignified inner and parenchymal with chloroplasts outer) in the central part of each leaf rib. The outer sheath of the vascular bundle was well expressed in representatives of D. antarctica originating from the islands Galindez, Winter, Yalour (genotype Y66), Lahille, and Rasmussen Point. The absence of the outer sheath or its deformation was observed in plants of genotypes Y67, Y62, S22, and DAR12. This may be due to the growth of the ancestral plants of these groups in the depths of the islands in a drier climate (Table 1). A well-developed parenchymal sheath is characteristic of plants from wet habitats (by the sea), while a less expressed parenchymal sheath of bundles is observed in plants with drier habitats, such as the Antarctic tundra (Romero et al., 1999). Parenchymal sheath formation is also associated with high insolation (Pyykko, 1966) and low temperature, possibly as a prerequisite for drought (Romero et al., 1999). It should be emphasized that the plants of the genotypes that we studied were grown for a long time in the same conditions in vitro. This complicates possible explanations of differences in the anatomical structure of D. antarctica owing to phenotypic response to the influence of growing conditions.
Compared to intact plants collected in the Antarctic, the metaxylem is reportedly better developed in the vascular bundles of all studied genotypes in vitro in the present study and genotypes D4 and D12 (from Galindez Island), BE1 (from Berthelot Island) from the previous study (Nuzhyna et al., 2019). It may be caused by the presence of higher temperatures in the laboratory compared to natural conditions. Vessels with small apertures are characteristic of plants growing in low temperature conditions, because water freezes more slowly in narrow vessels (Romero et al., 1999). Relatively narrower vessels were found in plants of genotypes DAR12, L59, Y62, and Y67 in vitro.
The presence of highly vacuolated motor cells (bulliform cells) is a xerophytic sign. The largest such cells were seen in genotype DAR12. Slightly smaller cells were present in plants of genotypes Y66 and Y67.
In our study, the in vitro plant cultivation took place under identical conditions. However, plant genotypes originating from different areas have different anatomical features. We can assume the fixation of certain signs in representatives of some genotypes at the genetic level, and more plastic phenotypic responses of other traits in other groups. We believe that the consolidation or absence of certain features depends on the individual degree of natural microenvironment. Such a dependence of adaptation features on microconditions has been demonstrated for D. antarctica plants in natural populations (Parnikoza et al., 2015, 2018). In particular, plants of the DAR12 and L59 genotypes are characterized by highly xerophytic traits under arid conditions with low temperatures (Table 1). These groups of plants were collected from the southernmost and most isolated islands. However, the results are divergent according to other studied parameters in most plants. This may indicate that the differences between groups are primarily due to growth conditions on a particular island. We found the parameters that are most influenced by external factors and may be fixed epigenetically, but do not change after long-term cultivation of plants in favorable culture conditions. These include the number of stomata, thickness of outer cell walls of the epidermis, expression of the parenchymal sheath of the vascular bundles, number of leaf ribs, and the presence of trichomes.
. Conclusion
There was no evidence of a clear dependence of the anatomical structure on the chromosomal status of plants of the studied genotypes. In general, in vitro plants of different genotypes had quite diverse morphometric epidermal parameters. It is possible that this heterogeneity is due to differences in the microconditions of the natural habitat of the original plants. External factors, such as temperature, water, salinity, high radiation, or influence of penguin colonies, act with varying intensity in different localities; the original plants thus take on epigenetically determined morphometric and anatomical diversity. Some of these features persist during in vitro clonal propagation.


