. Introduction
According to the most recently reported data (The Angiosperm Phylogeny Group, 2016; Lammers, 2007), the family Campanulaceae Juss. includes the following five subfamilies: Campanuloideae Burnett, Cyphioideae (A. DC.) Walp., Cyphocarpoideae Miers., Lobelioideae Burnett, and Nemacladoideae Lammers [see Cosner et al. (2004) and Takhtajan (2009) for another views]. Among them, the first subfamily is the best studied in a carpological manner (de Candolle, 1830, 1839; Dremliuha, 2013a, 2013b; Kolakovskiĭ, 1995; Zhinkina, 2010). In Campanuloideae, the following three tribes are distinguished: Cyanantheae Meisner, Wahlenbergieae Endlicher, and Campanuleae Dumortier (Hong & Wang, 2015). Tribe Cyanantheae is a sister clade to the joint clade of Wahlenbergieae and Campanuleae, but the relations within clades remain unresolved (Yoo et al., 2018). For example, an alternative view to the subfamily Campanuloideae is the recognition of only two clades within it. One of them corresponds to the first tribe, and the second one is considered as the alliance of the second and third tribes (Cosner et al., 2004; Eddie et al., 2003). The genus Jasione with a capitate pseudanthium and small reduced flowers (Sales et al., 2004) occupies an isolated position in Campanuloideae, and is placed either in the Campanuleae tribe (Cosner et al., 2004; Eddie et al., 2003) or in the Wahlenbergieae tribe (Roquet et al., 2009).
In Campanuleae, several monophyletic clades have been recognized, encompassing the polyphyletic Campanula L. and related genera. Among them, Campanula s. str.-clade included the genera Azorina Feer, Campanula, Diosphaera Buser, Edraianthus A. DC., Feeria Buser, Michauxia L’Hér, Symphyandra A. DC., Trachelium L., and Rapunculus-clade included the genera Adenophora Fisch., Asyneuma Griseb. et. Schenk, Campanula, Campanulastrum Small, Githopsis Nutt., Hanabusaya Nakai, Heterocodon Nutt., Legousia Durande, Physoplexis (Endl.) Schur, Phyteuma L., Petromarula Hedwig f., Triodanis Raf. (Eddie et al., 2003; Roquet et al., 2008, 2009).
In all clades of the Campanuloideae subfamily, a considerable diversity of fruit structure is observed, exhibited by fruit wall consistency, dehiscence type, carpel number, ovary insertion, and seed number (Lammers, 2007). This diversity is a result of complicated and multiple changes pertaining to the gynoecium and fruit evolution. For this reason, consideration of carpological characters has remained controversial in family-level systematics during the nineteenth–twentieth century (see review by Andreychuk & Odintsova, 2020).
In the Cyanantheae, the gynoecium is isomerous, and the carpels are antepetalous (Lammers, 2007). The ovary is usually inferior, in few species, it is semi-inferior or almost superior. In Cyanantheae and Wahlenbergieae, the fruits are predominantly loculicidal capsules, dehiscent by apical valves, or are recognized as a berry in the former. In Wahlenbergieae, the fruit sometimes dehisces by pores or an operculate capsule. In Campanuleae, the fruit is usually a poricidal capsule, dehiscent by lateral pores or fissures, or is indehiscent in certain cases (Hong & Wang, 2015; Lammers, 2007). The carpel number in Campanuloideae varies from two to five or more.
In many genera of the Campanuleae tribe, a unique capsule with an axicorn has been elucidated by Kolakovskiĭ (1985, 1995). Anatomical studies have revealed that a tissue of axicorn is composed of roundish cells with thick lignified cell walls (Lakoba, 1986). Dehiscence in Campanula and related genera proceed through hygroscopic movements of the axicorns, resulting in the formation of openings in the ovary wall.
In our previous studies (Andreychuk et al., 2020; Andreychuk & Odintsova, 2019), we analyzed the inner structure and dehiscence of capsular fruits in two species of Campanuleae, namely Campanula latifolia L. (Campanula s. str.-clade) and Asyneuma canescens (Waldst. & Kit.) Griseb. & Schenk (Rapunculus-clade), and subsequently revealed a profound similarity in the formation of openings in the wall of the capsule by the axicorn. Therefore, we considered the axicorn as a histological structure inside the ovary, composed of lignified parenchyma of the septa, and composed of the ovary wall in certain cases, which underwent early differentiation during flower–fruit morphogenesis. Our definition of axicorn was similar to that proposed for the Campanula capsule by Roth (1977), who did not use the term “axicorn,” than to the definition suggested by Kolakovskiĭ (1995), who named the axicorn a “specialized organ” inside the ovary, which could be responsible for seed dispersal.
Based on Kolakovskiĭ (1995) data on fruit morphology in representatives of the different tribes of Campanuloideae, we aimed to compare the anatomy and micromorphology of the fruiting ovary in nonaxicorn and axicorn-bearing capsules with different modes of dehiscence, i.e., by apical valves or by lateral pores.
For the present study, we selected the following three ornamental representatives of the subfamily Campanuloideae: Platycodon grandiflorus (Jacq.) A. DC. (tribe Cyanantheae), Jasione montana L. [tribe Wahlenbergieae; but see Eddie et al. (2003) and Cosner et al. (2004)] and Adenophora liliifolia (L.) A. DC. (tribe Campanuleae). All studied species were herbaceous annual, biennial, or perennial herbs (Lammers, 2007) and have been reportedly used as medicinal plants. The last two species belong to the native flora of Ukraine (Mosyakin & Fedoronchuk, 1999) and are also cultivated as ornamental plants. Platycodon grandiflorusis widely cultivated in the botanical gardens of Ukraine as an ornamental plant (Catalogue of ornamental herbaceous plants of botanical gardens and arboretums of Ukraine, 2015); its cultivars vary in flower color and size and contain components which possess antitussive, antitumor, and antioxidation properties (Hawke, 2009; Ji et al., 2020). Jasione montana has a wide range of medicinal properties (Hrodzinsʹkyĭ, 1992). Adenophora liliifolia is widely used as an ornamental garden plant, while many cultivars of different species of Adenophora are grown for medicinal use (Andreeva et al., 2008). In the studied genera, secondary pollen presentation was described as typical and characterized by a long hairy region of the style (Leins & Erbar, 2006).
. Material and Methods
Plant Material and Collection Sites
The flowers and fruits of P. grandiflorus were sampled from the collection “Medicinal Plants” of the Botanical Garden of Ivan Franko National University of Lviv (49°49′47″ N, 24°3′40″ E, elevation: ±320 m a.s.l.). The inflorescences and fruits of J. montana were collected from the population in a pine forest near the village Arlamovskaya Volya in the Mostysky district of the Lviv region (49°50′55″ N, 23°14′37″ E, elevation: ±220 m a.s.l.). The flowers and fruits of A. liliifolia were collected at the summit of Kasova Mountain (near the viewing platform) in the Halych district of the Ivano-Frankivsk region (49°13′38″ N, 24°41′48″ E, elevation: ±311 m a.s.l.). The fresh material was fixed in 70% ethanol and sectioned for an anatomical survey, while dried fruits were stored in cardboard boxes for observations of the structure of dehiscent fruit. We investigated the structure of the flower and fruit of each species at different stages of development, namely floral bud (preanthetic stage), flowering (anthesis), development of the fruit (postanthetic stage), and dehiscence of the fruit. We performed sampling for n = 5–6 floral buds, n = 5–6 flowers, and n = 10 fruits for each species from 5 to 10 individuals randomly selected.
Microtechnical and Microscopical Methods
The morphology of each fruit was examined via transverse and longitudinal dissection of the fixed materials with a razor blade. We used a stereo-microscope MBS-10 (Lomo; USSR), and the measurements were performed using a vernier caliper (Tricle; PRC). The morphological descriptions of the fruits were provided according to the terminology adapted from Fedorov and Artiushenko (1975) and Artiushenko and Fedorov (1986).
The anatomical structure of each fruit was examined on transversal and longitudinal sections of the fixed materials obtained using a razor blade and after the samples were mounted using water. Tissue lignification was determined based on the phloroglucinol test reaction (Barykina et al., 2004). The anatomical sections were examined using the XS-2610 light microscope (MICROmed, PRC). The AmScope MD digital ocular and the AmScope 3.7 software (AmScope, PRC) were used to acquire images of the sections.
. Results
Platycodon grandiflorus
Morphological Structure of Flower and Fruit
The flower of P. grandiflorus was 4 cm in length and 2–3 cm in diameter in average; it was pentamerous and upright (Figure 1A). Five (six in certain cases) calyx lobes (or sepals) were observed to be lanceolate, elongated, entire, directed upwards, 5–6 mm in length, and 2–3 mm in width. The corolla was widely bell-shaped with triangular lobes. The stamens presented with long anthers. The filaments at the base were found to be widened and pubescent. The flowers in our material had only one corolla whorl, in contrast to Eichler’s (1875) monograph, where two corolla whorls were reported.
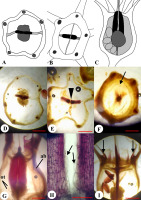
Figure 1
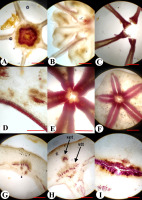
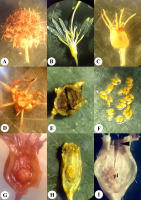
Morphological structure of the flower and fruit of Platycodon grandiflorus: General morphology (A–I) and dissected fruits (J–O). General view of the flowers at anthesis and early postanthesis (A); stigma, style, and superior region of the ovary in the floral bud (B) and the opened flower (C); fruit before dissemination (D) and after dissemination (E); seeds with narrow wing (F); dry fruit after calyx lobes fell off (G); opened fruit (the light surface of fruit valves after separation of tissues is visible) (H); top view of the opened fruit (I); longitudinal section through the young fruit (J), dry fruit (K), and opened fruit with distant valves (L) [central column (cc) and placenta (pl) are visible]; transverse section at the level of calyx lobe insertion (M); transverse section above the placentae level (N); placentae in transverse section (O).

The ovary of P. grandiflorus was observed to be semi-inferior, 1 cm in length and 1 cm in diameter, obconic, and presented with the following two regions: the superior one was situated between the insertion of the calyx lobes and the style base, which exhibited the form of a pentahedral pyramid (Figure 1B,C), and the inferior region was situated between the insertion of the calyx lobes and the ovary base (pedicel). In the preanthetic flower, the style was short, and the stigma lobes were erect and closed together (Figure 1B), while at anthesis, it was observed to be elongated and filamentous with five descended stigma lobes (Figure 1C).
The fruit of P. grandiflorus was observed to be semi-inferior, five-locular (six-locular in certain cases), multiseeded, dry capsule (Figure 1D,G). The capsule was 1.5–1.7 cm in length and 1.0–1.5 cm in diameter. It was upright, with appearance ranging from light yellow to dark brown, transitioning into a dark brown-colored structure, and was observed to be spotted when dried. The surface of the fruit was smooth with 10 protruding ribs. During fruit development, the corolla dried, curled, and fell off, as observed similarly in the case of the stamens and style. The calyx lobes also fell off at subsequent stages.
The internal structure of the fruit differed in the superior and inferior regions. The superior region (above calyx lobes) in the cross sections was pentagonal, and the septa were wedge-shaped, narrowed towards the center (Figure 1M, Figure 2A–C). In the inferior region (below calyx lobes), the capsule was round and thin-walled in the cross sections, and locules were separated by long, thin septa (Figure 1N, Figure 2D–G). There were small placentae at the mid-level of the inferior region of the fruit (Figure 1J,O, Figure 2G). The lobes of the placentae were divided and bent upward and downward. In the central part of the fruit, a central column (Figure 1K) was observed to be rounded in cross section (Figure 1K,L). The central column integrally extended from the ovary base to the placentae, while the septa above this juncture detached in the center from each other (Figure 1L).
Figure 2
Series of transverse sections of the fruit of Platycodon grandiflorus at different levels (A–F) and longitudinal section (G) where levels of sections from A–F are indicated; left half of G illustrates the septa plane and right half depicts the locule plane. Main vascular bundles are depicted, and lignified layer of the fruit wall is indicated in solid black.
Seeds were 2.0–2.5 mm in length and 1.0–1.5 mm in width, ovate, flattened, smooth, shiny, with a narrow wing on one side, and exhibited light to dark brown (Figure 1F) coloration. The capsule contained on average 128 seeds (n = 10, minimum = 75, maximum = 160).
Anatomy of Fruit
Unripe green fruit of P. grandiflorus presented with a fleshy consistency, and the exocarpium was unlignified, unilayered, and glabrous. The mesocarpium in the middle and the basal part of the fruit consisted of 18–20 layers of cells, was thickened in the ribs, and was unlignified at anthesis and early postanthesis. When the fruit was at a maximum size but remained green and fleshy, the reaction with phloroglucinol showed the presence of tissue lignification in the cell walls of several layers of subepidermal parenchyma evenly lining the entire surface of the locules (Figure 2, Figure 3C–F). The lignification occurred in the fruit wall and septa. Only in the location of the dorsal bundles in the superior region of the ovary was lignification observed to be interrupted (Figure 4A). In the inferior region of the fruit, the number of layers of lignified tissue was one–two (Figure 3C,D); however, in the superior region, it increased up to eight–nine layers (Figure 4). The maximum concentration of lignin (revealed by intensity of the color) was observed in the proximal parts of the septa, where the lignified areas of neighboring septa were connected (Figure 3E,F). Lignified cells demonstrated an isodiametric roundish form in the transverse section and extremely thick walls. The endocarpium was unilayered and unlignified (Figure 4B). The vascular system of the fruiting ovary in P. grandiflorus consisted of the following 10 ascending vascular bundles in the ovary wall, which passed along the ribs: Five vascular bundles were located on the septa radii (septal veins), and the other five vascular bundles were located on the median planes of the carpels (dorsal veins) (Figure 2, Figure 3G,H). The septal veins left the inferior region of the ovary wall and entered the calyx lobes, branching onto the calyx lobe trace (sepal trace) and each of the stamen traces (Figure 3I). The dorsal veins were divided into the corolla lobe trace (petal trace) and the carpel dorsal bundle. In the superior region of the ovary, there were numerous small bundles in the septa (Figure 3F), and such bundles were not present in the inferior region of the ovary. In the central column of the ovary, the vascular cylinder was divided into five bundles on the septa radii (Figure 3A–C), which supplied the placentae and the ovules. These bundles entered the style in the ovary top, as observed for the dorsal carpel bundles, too (Figure 2A).
Figure 3
Anatomical structure of the fruit of Platycodon grandiflorus in transverse section: Central column with a circle of vascular tissue (A); the placental region of the fruit and weak lignification of the septa are visible (B); the central part of the fruit above the placentae (C); fruit wall in the inferior region of the ovary with a thin layer of lignified parenchyma in the mesocarpium (D); the central part of the fruit in the superior region (E); the superior region of the ovary with a thick layer of lignified parenchyma in the mesocarpium and the septa detached from each other (F); vascular bundles in the inferior ovary wall at early postanthesis (G–I); dorsal vein (G), septal vein (H), and stamen trace (I); set – calyx lobe trace (sepal trace); stt – stamen trace. Scale bars = 1 mm.
Dehiscence of Fruit
Dehiscence of the fruit of P. grandiflorus occurred longitudinally after the style fell off. Initially, the superior region of the fruit gradually split basipetally into five valves along the mid planes of carpels to the level of calyx lobe insertion (Figure 1E). Then, the valves separated from each other and bent outward, forming an opening to release seeds when shaken (Figure 1H,I). The dorsal carpellary vein split into two halves when the fruit dehisced. In a dry unopened fruit, the septa were already observed to be separated in the center along ventral sutures, allowing the valves to descend from the center (Figure 3C,E,F). During the drying of the fruit, the valves descended from each other to the level of the placentae (Figure 1L). Below the placentae, the central column remained intact. In a completely dried and opened fruit, the external parenchymatous tissue of the valves dissected from the lignified tissue (Figure 1H). Thus, the fruit of P. grandiflorus was dehisced by the separation of the septa from each other, in the center of the fruit along the ventral sutures, to the level of the placentae, and loculicidally via five valves, which bent outwards in the superior region of the fruit to the point of calyx lobe insertion.
Jasione montana
Morphological Structure of Flower and Fruit
The flowers of J. montana were arranged in a head-like pseudanthium surrounded by involucral bracts (Figure 5A). The inflorescence consisted of approximately 87 flowers (n = 50, minimum = 41, maximum = 143) depending on the branching order of the shoot. The order of flower maturation in the heads was centripetal. The flowers were epigynous, pentamerous, with long peduncles, and were spirally arranged on the common receptacle. The inferior ovary was ovoid, 1.0–2.0 mm in length, 1 mm in diameter, and was green in color. The calyx lobes were long, awl-like, and 2 mm in length. The corolla was dissected to the base with linear lobes. The stamens were fused around the style, resulting in the formation of a tube. The style was long, cylindrical, and the stigma was two-lobed (Figure 5B).
Figure 5
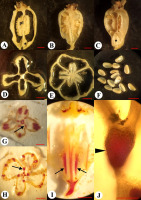
Morphological structure of the flower and fruit of Jasione montana in the fixed material: Fruiting pseudanthium (A), flower (B), and fruit (C); apical view of the dry capsule at the beginning of dehiscence (D), full dehiscence (E), and seeds (F); longitudinal sections of young fruit (G), dry fruit (H), and opened fruit (I) [spherical placenta (pl) and remnants of a style (arrowhead) are visible].
The dry fruit of J. montana was an inferior, bilocular, ovoid, dry capsule, 2.5–3.0 mm in length, and 1.5–2.0 mm in diameter (Figure 5C). The capsule was upright. The fruit color ranged from yellow to light brown. The surface of the fruit was smooth, with the presence of five protruding ribs. The fruit walls were thin and transparent, and the seeds could be visible inside. The superior region of the ovary was thin and flat (Figure 5G). The calyx lobes were persistent. After anthesis, the corolla lobes, stamens, and style gradually dried, twisted, and fell off. The scar of the fallen style was the location where the dehiscence of the fruit commenced (Figure 5I).
In the fruiting ovary, one septum divided the ovary into two locules (Figure 6A). In places of attachment to the fruit wall, the septum was thin distally but showed marked density. In the basal part of the ovary, there was a thin central column bearing a spherical placenta covered with ovules (Figure 5G, Figure 6A–D). The septum above the placenta was disconnected longitudinally in the center along the ventral sutures of the carpels (Figure 5H, Figure 6E).
Figure 6
Micromorphology and anatomy of the fruit of Jasione montana: Schematic representations of transverse sections of the fruit at the placentae (A) and above the placentae level (B); longitudinal section showing lignified layer of the fruit wall as indicated in solid black (C); phloroglucinol reaction in transverse sections of the fruit at placentae (D), above placentae (E), and at the level of the superior part of the ovary (F); ventral suture shown by arrowhead (E); open median slit is marked by arrow (F); phloroglucinol reaction in longitudinal sections of the fruit in the plane of septum (ab – ascending bundle; ot – ovule trace) (G); a close-up view of the septum in the open fruit showing unlignified endocarpium indicated by arrows (H); tangential section in the median carpel plane: Stamen traces are indicated by arrows (I). Scale bars = 500 µm (A–G,I); 200 µm (H).
The seeds were observed to be small, 0.5–0.6 mm in length and 0.2–0.3 mm in width, elliptical or ovoid, shiny, and the coloration ranged from light brown to dark brown (Figure 5F). The capsule contained on average 37 seeds (n = 20, minimum = 6, maximum = 46). The number of seeds in capsules from an axile inflorescence was 25% less than that in the capsules from an apical inflorescence.
Anatomy of Fruit
The exocarpium was unilayered and unlignified, and in certain cases, it was covered with simple unicellular trichomes. The mesocarpium consisted of six–seven layers of unlignified cells. The endocarpium was also unilayered and unlignified. The septum had five–seven cell layers; in dry fruit, thickness increased to a certain extent and lignification was observed in the proximal parts above the placenta (Figure 6D,E,G). Lignified cells exhibited isodiametric form on transverse sections and lengths were 4–5 times greater on longitudinal sections. However, the epidermis, which covered the septum, was not lignified (Figure 6H). In dry fruit, the uppermost portion of the septum was composed of the lignified parenchyma proximally and two epidermal layers distally, while unlignified parenchyma of the septum was absent. The style base was also lignified in fruit (Figure 6I).
The fruit wall of J. montana contained five unbranched ascending vascular bundles on the calyx lobe radii, and none of them located on the septum plane (Figure 6D,E). Traces of corolla lobes, stamens, and the dorsal vascular bundles of carpels branched in the superior region of the ovary from ascending bundles (Figure 6I). Vascular tissue from the peduncle entered the central column and placenta, wherein ovule traces were formed (Figure 6G).
Dehiscence of Fruit
By the time of seed release, the septum above the placenta split vertically into two halves along the ventral suture of the carpels (Figure 5H,I). Simultaneously, in the superior region of the ovary, a transverse slit was formed along the median plane of the carpels (perpendicular to the septa), beginning at the style scar (Figure 5D,E, Figure 6F). The slit was located horizontally across the entire superior region of the ovary. When the capsule dried, the slit in the ovary roof widened, separating the halves of the septum from each other. Thus, the fruit of J. montana dehisced by the separation of the septum halves above the placenta, along the ventral sutures, and loculicidally in the superior region of the ovary.
Adenophora liliifolia
Morphological Structure of Flower and Fruit
The flowers of A. liliifolia were 1.5 cm in length and 1 cm in diameter, pentamerous, and pendent during flowering (Figure 7A). Calyx lobes were five–six, serrate, 3 mm in length and 1–2 mm in width, were directed in a downward direction at preanthesis; however, they were observed to be in an upward direction at anthesis and presented a downward direction again at postanthesis (Figure 7D–F). The corolla was bell-shaped, and the stamens demonstrated long anthers. The stamen filaments at the base were slightly expanded and pubescent. The ovary was inferior, 2.5–3.0 mm in length and 1–2 mm in diameter. The style was long, exerted from the corolla on 0.5–1.0 cm, and the stigma was three-lobed.
Figure 7
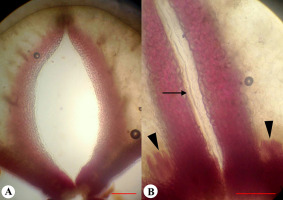
External morphology of the flower and fruit of Adenophora liliifolia: Fragment of inflorescence at the flowering and fruiting stages (A); fruit before dehiscence (B), and at the dehiscent stage (C). Fruit in natural orientation: Ovary at preanthesis (D), anthesis (E), and fruiting (F). Development of depression prior to opening indicated by an arrow; a close-up view of hippocrepiform slit in the fruit wall (G,H); lignified strand is marked by an arrow (G).

The dry fruit of A. liliifolia was an inferior, multiseeded, dry capsule with three–four locules, 7–9 mm in length, and 4–7 mm in diameter. The capsule was pendent and obovate (Figure 7B). The fruit color ranged from light green to dark green. On the surface of the fruit there were six–seven prominent ribs, of which a few were branched. During fruit-drying conditions, the corolla dried, curled, and fell off (Figure 7C). The stamens, style, and calyx lobes also fell off later. At preanthesis, in the basal part of the ovary wall, small diamond-shaped depressions were visible between the ribs, and in terms of number, they were present according to the number of locules (Figure 7D). Depressions were formed on the septa radii, where bundles passing the ribs were more distant from each other. The depressions became deeper at anthesis and postanthesis, indicating the formation of future openings (Figure 7E,F, Figure 8A,C).
Figure 8
Micromorphology and anatomy of the fruit of Adenophora liliifolia. Longitudinally dissected fruits (A–C): Tangential (A,C) and radial sections (B) [placenta (pl) and basal depressions are visible (asterisks)]; the lignified strand is marked by using a dashed line (B). Transverse section at the depressions (D) and placentae levels (E); seeds (F); phloroglucinol reaction in transverse (G,H) and longitudinal (I) sections of the fruit (the lignified strands are indicated by arrows); a close-up view of the lignified strand in septa (J) (unlignified endocarpium is indicated by an arrowhead). Scale bars = 2 mm (A–I); 500 µm (J).
The capsule was observed to be round on the upper part of the cross sections and presented with three or four lobes at the base through markedly shortened septa (Figure 8D,E). In the middle part of the fruiting ovary, there were placentae with lobes bent in the upward and downward directions. The placentae split longitudinally and demonstrated wavy edges (Figure 8A). In the central part of the fruit, there was a central column, a diamond-shaped structure observed in the cross section view (Figure 8D). The central column integrally extended from the ovary base to the base of the placentae. Above the middle-height of the fruit, the septa were observed to be thin-walled and incomplete from the placentae to the superior region of the fruit (Figure 8, Figure 9E). Seeds were 1.5 mm in length and 1.0 mm in width, oval, slightly flattened, smooth, shiny, with a wing on one side, and exhibited coloration ranging from light brown to dark brown (Figure 8F). The capsule contained on average 130 seeds (n = 10, minimum = 105, maximum = 153).
Figure 9
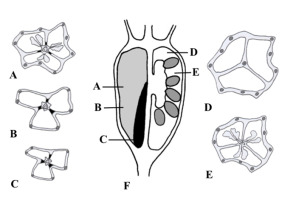
Series of transverse sections of the fruit of Adenophora liliifolia at different levels (A–E) and longitudinal section (F) where levels of the sections from A–E are indicated; left half of F illustrates the septa plane and right half depicts the locule plane. Main vascular bundles are depicted, and lignified layer of the fruit wall is indicated in solid black.
Anatomy of Fruit
The exocarpium was unlignified and consisted of one layer of cells. In certain cases, there were small, spiny hairs present in the depressions. The hairs were unicellular and unlignified. The mesocarpium was unlignified and consisted of 10–16 layers of cells. The endocarpium was unilayered and unlignified. In the basal part of the fruit, from the base to the middle part of the placenta, in the septa, there were strands of thick-walled tissue. These strands attached to the central column along its length (Figure 8B,I, Figure 9F). In the cross section view, the strands exhibited a trapezoidal or oval shape. The reaction test performed for the lignified tissues showed that the strands were composed of cells with lignified walls. The cells of the epidermis, which covered the strands, were small and unlignified (Figure 8J).
The fruit wall of A. liliifolia contained three–four ascending vascular bundles on the locule radii and these bundles split above on 10–12 main vascular bundles, and they were located in the ribs and gave rise to multiple small anastomosing bundles (Figure 8G,H, Figure 9A–E). In the superior region of the fruit, traces of calyx lobes, corolla lobes, stamens, and dorsal bundles of the carpels formed from the ascending vascular bundles. In the central column, at the fruit base, a triangular or diamond-shaped vascular cylinder was observed (Figure 8G). Toward a higher location, the vascular cylinder split into three or four vascular bundles on the septa radii. These vascular bundles supplied the placenta and ovules (Figure 8H).
Dehiscence of Fruit
Fruit dehiscence proceeded with the formation of hippocrepiform opening on the lower outline of each depression on the basal part of the ovary wall. This opening was 2 mm in length and 1.5 mm in width. As the fruit was pendent, the lower outline of the opening was observed to be in the upper position (Figure 7C,G,H). Later, the fragment of the fruit wall, outlined by the slit, bent outward, facilitating the release of the seeds when shaken by the wind. Then, when the opening became larger, the fruit septa detached from the central column along the lower edge of the lignified strands in an acropetal direction. Finally, when the covering fragments of the fruit wall were curled up, lignified strands of septa on the inner surfaces were visible (Figure 7G).
. Discussion
Morphological Structure of Fruit and Adaptations to Dehiscence
The three studied species, P. grandiflorus, J. montana, and A. liliifolia, differed in the merosity of the gynoecium, ovary position, and orientation. A pentamerous (or isomerous, considered in a wider sense) gynoecium, as observed in P. grandiflorus, occurs in each tribe of Campanuloideae (Lammers, 2007) and seems to be an ancestral condition from the evolutionary morphology viewpoint, while an oligomerous gynoecium, composed of two or three carpels, is deemed a derived condition. Dimerous gynoecium occurred in J. montana, where it corresponded with the general level of flower reduction in a condensed pseudanthium (Lammers, 2007; Sales et al., 2004). The other characters correlated with this reduction include the small size of the flowers and the dissected corolla. The trimerous gynoecium in A. liliifolia is typical for Campanula-related genera and all members of Campanuloideae (Lammers, 2007).
In P. grandiflorus the ovary develops into a semi-inferior fruit with an upright orientation. In the tribe Cyanantheae, semi-inferior fruits are formed in Campanumoea Blume, Codonopsis Wall., Cyananthus Wall., and Cyclocodon Griff. ex Hook. f. et Thoms. (De Wilde & Duyfjes, 2012). In the tribe Wahlenbergieae, they are characteristic of Wahlenbergia Schrad. ex Roth (Lammers, 2007). The inferior ovary is considered a derived character state; however, there were revealed certain cases of reversals from an inferior state to a semi-inferior or superior state (Basso-Alves et al., 2017; Rudall, 2002; Soltis & Hufford, 2002). In this connection, the semi-inferior ovary insertion in Campanuloideae still needs to be properly evaluated as ancestral or derived condition.
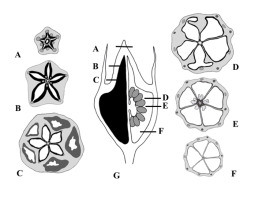
In the three species, we revealed the following two vertical zones present inside the ovary according to the findings reported by Leinfellner (1950): synascidiate and symplicate. In the first zone, a central column with a vascular cylinder existed, and the locules were completely separated by thin septa. Takhtajan (2009) suggested that the Campanulaceae possesses a paracarpous gynoecium with thick, expanded placentae, forming false septa and pseudoaxile placentation. Thus, the ovary is subdivided into pseudoloculi, and a unilocular paracarpous gynoecium leads to the development of the multilocular paracarpous gynoecium. In contrast, Bobrov et al. (2009) defined such a gynoecium in the Campanulaceae as a phragmocarpous gynoecium, i.e., a secondary-multilocular, paracarpous gynoecium with parietal placentation simulating axile placentation.
The placentae in two of the studied species were located in the middle of the locules; however, in J. montana, it was located in the lower position. The placenta in A. liliifolia was clearly parietal and located in the symplicate zone. The bilobate placentae in P. grandiflorus were axile below and were parietal above, while a spherical placenta in J. montana is observed to be of an axile origin. The condition, wherein the placenta of the syncarpous gynoecium is axile below and is parietal above, occurs widely in angiosperms and corresponds to the frequent combination of the synascidiate and symplicate zones in the ovary (Leins & Erbar, 2010; Shamrov, 2012; Takhtajan, 2009).
The position of the placenta in P. grandiflorus and J. montana indicated demarcation of a boundary between the synascidiate and symplicate zones, evident by the basal endings of the ventral slits during fruit dehiscence. There were no ventral sutures of the carpels in the synascidiate zone, and the dehiscence slits were terminated here. Both species were similar in the upright flower–fruit orientation, in contrast to A. liliifolia with pendent flowers and fruits. As many Campanuloideae members with capsular fruits are ballistochorous, fruit dehiscence proceeds apically in erect fruits and basally or laterally in pendent fruits (de Candolle, 1830; Levina, 1957; Niu et al., 2016; van der Pijl, 1982). Both modes, erect and pendent, are present in Campanuleae, and particularly in Campanula. In Campanula section Medium, the flowers become pendent during fruiting, while in Campanula section Rapunculus, fruits are erect (Dremliuha, 2013a, 2013b). Based on this characteristic, A. liliifolia with pendent fruits and basal openings resembles species of Campanula section Medium, for example, C. latifolia (Andreychuk & Odintsova, 2019). However, according to molecular phylogenetic data (Mansion et al., 2012; Roquet et al., 2008; Xu & Hong, 2020), it is inferred that Adenophora is not closely related to species of Campanula s. str.-clade, where C. latifolia has been placed. Therefore, the fruit orientation and openings position seem to be a homoplastic character in the Campanuleae.
Anatomical Adaptations to Fruit Dehiscence
Anatomical features of the ovary and fruit in the Campanuloideae have been poorly studied previously. Fruits in P. grandiflorus and J. montana were glabrous, whereas in those of A. liliifolia, the exocarpium was covered with unicellular hairs, the most common trichome type occurring in the Campanuloideae (Lammers, 2007). The fruit wall of the studied species was composed of chlorophylous parenchyma at anthesis and underwent subsequent histological differentiation to a certain extent. In P. grandiflorus, the fruit wall bore lignified parenchyma in the inner zone of the mesocarpium and in a subepidermal position in the septa from the fruit base to the top. The lignified zone was more prominent above the calyx lobe insertion, where dehiscence occurred. It was noted for the first time that the proximal ends of incomplete septa in the symplicate zone were composed of cells with robust lignified walls.
According to our observations in J. montana, the fruit wall did not contain any lignified cells, except tracheal elements of vascular bundles, as observed in A. liliifolia. In both species, lignification was revealed only in cells of proximal regions of septa. In A. liliifolia, the lignified parenchyma was observed as a narrow strand in each septa and was named “axicorn” by Kolakovskiĭ (1995). Cells with lignified cell walls exhibited a roundish form in the transverse section, and no difference in cell orientation was revealed. In the three species, as in Campanula latifolia (Andreychuk & Odintsova, 2019) and Asyneuma canescens (Andreychuk et al., 2020), the endocarpium was found to be unlignified.
Based on the application of the morphogenetic approach proposed by Bobrov and Romanov (2019) to fruit wall anatomy, we classified the capsule in P. grandiflorus as Forsythia-type, i.e., a sclerenchymatous layer was localized in the inner zone of the mesocarpium, while the endocarpium remained parenchymatous. The fruit in J. montana and A. liliifolia did not correspond to this type due to a lack of lignification in the fruit wall. Instead, fruit dehiscence in J. montana and A. liliifolia was facilitated by mechanical tissue in the septa. Based on this phenomenon, we recognized a new type of capsule, a Campanula-type that presented without mechanical layers in the fruit wall but contained lignified tissue in the septa, which further influenced dehiscence. For the first time, we described an anatomical structure of such a capsule in Campanula latifolia (Andreychuk & Odintsova, 2019), where lignification spread slightly to the fruit wall in the lower part of locules. In J. montana, A. liliifolia, and in Asyneuma canescens, no occurrence of lignification of the fruit wall was observed.
The capsule of the Campanula-type differs from the capsule of the Galanthus-type (where sclerenchyma does not comprise a continuous topographic zone in any histogenetic zone of the fruit wall) by the presence of lignified strands or continuous zones of lignified cells in the septa, whereas in Galanthus, no lignified zones could be observed in the entire fruit (Rasmussen et al., 2006). One may speculate that the capsule of the Campanula-type may be derived from the Forsythia-type of the capsule of P. grandiflorus because of parenchymatization of the fruit wall. Based on our assumption, from the capsule of the Campanula-type, fleshy capsules or inferior berries could originate because of complete parenchymatization of the septa.
In the middle zone of the mesocarpium, the appearance of a ring of ascending vascular bundles could be noted. In P. grandiflorus and A. liliifolia, there were approximately 10 vascular bundles on the calyx lobe and corolla lobe radii, with numerous branches. In J. montana, only five antesepalous bundles could be observed owing to general flower reduction. In the central column of the studied species, the vascular cylinder underwent division into complex ventral bundles (one in each septum), thus supplying the ovules. No trans-septal bundles emerged in the inferior portion of the ovary. Thus, we defined the ovary wall and ovules as structures exhibiting distinct innervation.
Classification of Fruit Dehiscence
We used information on the detailed classification of fruit dehiscence reported by Kaden (1962, 1964, 1965) for investigating fruit dehiscence in the studied species. Kaden (1965) accurately defined the fruit in J. montana as a syncarpous, bilocular, inferior capsule with incomplete dorsiventral dehiscence. According to our data, this definition could also be considered acceptable for P. grandiflorus, except that the capsule was semi-inferior and pentamerous.
Dehiscence of fruits in many Campanuloideae was precisely studied by Kolakovskiĭ (1995), who established a “carpological” system for tribes and subfamilies of Campanuloideae (considered as Campanulaceae by Kolakovskiĭ).
According to Kolakovskiĭ (1995), genera that currently belong to the tribes Cyanantheae and Wahlenbergieae presented previously with nonaxicorn, dehiscent, or indehiscent fruits, while most members of the tribe Campanuleae exhibited axicorn-bearing capsules. The fruit type in Platycodon and Jasione was defined by Kolakovskiĭ (1995) as a nonaxicorn, valvate capsule with apical valves.
Fruit in Adenophora was defined by Kaden (1965) as a fruit in Campanula – a syncarpous capsule with hippocrepiform dehiscence. Earlier, Kaden (1964) noticed and reported that hippocrepiform fruit dehiscence in Campanula occurred on the septa radii, in contrast with the Papaver fruit that demonstrated a hippocrepiform opening on each carpel.
Kolakovskiĭ (1995) studied a fruit of Adenophora stenanthina (Ledeb.) Kitag. (in the original work, referred to as Adenophora marsupiflora Fisch.), and classified it as an axicorn-bearing fissuricidal capsule, with persistent calyx lobes, thin walls, and small basal axicorns declining from the central column.
Following Kaden (1962), we intended to highlight all types of slits observed during fruit dehiscence in A. liliifolia. Except for hippocrepiform slits on the fruit wall, other slits separating septa from the central column occurred in place of flaps, i.e., septifragal slits.
Based on our experience, we suggested an additional principle to the classification of fruit dehiscence (Odintsova, 2016) to determine whether dehiscence slits developed only in the carpel tissue or in the tissue of an inferior ovary wall. The last one is considered to be of a complex origin. A complex origin would involve receptacular or perianth tissue outside and carpellary tissue inside, or formation of the tissue would result from peripheral upgrowth of an undifferentiated floral apex below the insertion of perianth members (Leins & Erbar, 2010; Soltis & Hufford, 2002). In any case, the identity of the inferior ovary tissue is not the same as that of a carpel tissue. Therefore, in semi-inferior and inferior fruits, dehiscence may occur above the insertion of the calyx lobes, i.e., in the superior region of the ovary (suprasepalous), as in the superior fruits, in the carpellary tissue only. This mode of dehiscence was revealed in P. grandiflorus and J. montana. The other mode was observed in Campanuleae, where dehiscence slits occurred laterally, below the insertion of the calyx lobes, in the inferior region of the ovary (infrasepalous), as was revealed in Campanula latifolia (Andreychuk & Odintsova, 2019), Asyneuma canescens (Andreychuk et al., 2020), and in Adenophora liliifolia.
. Conclusions
Using light microscopy methods, we revealed micromorphological and anatomical characters of the gynoecium and fruit of ornamental P. grandiflorus, J. montana, and A. liliifolia. All studied species presented with synascidiate and symplicate zones in the ovary. Platycodon grandiflorusand J. montana demonstrated erect fruits with axile or mostly axile placentae and superior, incomplete dorsiventral dehiscence. Adenophora liliifoliais characterized by pendent fruit, parietal placentae, and inferior, hippocrepiform-septifragal, interlocular dehiscence. In P. grandiflorus, the septa and fruit wall were composed of lignified parenchyma in subepidermal layers, while in other species, lignification was evident only in proximal portions of the septa (J. montana) or was observed as a narrow strand known as an axicorn (A. liliifolia). Unlignified endocarpium was revealed in all the studied species. In J. montana and A. liliifolia, a new histogenetic type of capsule was described as Campanula-type, exhibiting no lignified layers in fruit wall but containing lignified tissue in septa, thus influencing dehiscence. The three studied ornamental species represented two main types of capsule in Campanuloideae, depending on whether the superior or inferior region of the fruit provided the opening.