. Introduction
Comfrey (Symphytum officinale) is a common wild plant, a member of the Boraginaceae family. Its root (Symphyti Radix) contains high concentrations of allantoin and is an important plant source of this compound. In addition, it contains phenolic acids, flavonoids, glycopeptides, tannins, triterpene saponins, and alkaloids. The use of preparations containing comfrey root is based on its anti-inflammatory, analgesic, and astringent effects. For centuries, it has been used externally as a compress on wounds or burns and in the treatment of osteoarthritis, bruises, back pain, and skin irritations. Moreover, comfrey-containing preparations reduce swelling and relieve pain associated with injuries. It has been demonstrated that comfrey root extract has a proliferative effect and stimulates tissue regeneration. Allantoin, mucilage polysaccharides, and rosmarinic acid are of key importance for their pharmacological properties (Dinica et al., 2021; Dresler et al., 2017). However, due to the content of hepatotoxic pyrrolizidine alkaloids, comfrey has been withdrawn from products for internal use. It is still used as an ingredient in ointments, creams, and other moisturizing cosmetics, and there are no reports of toxic properties of these products (Mazzafera et al., 2008).
Allantoin is commonly used as an ingredient in many different types of cosmetic and pharmaceutical products, acting as a skin protector in concentrations ranging from 0.5 to 2%. It belongs to the group of ureides and is an odourless white powder with an acidic pH, soluble in slightly polar solvents, water and alcohol, and easily biodegradable. Allantoin can form complexes with many organic and inorganic substances as well as salts with similar properties and activity (Lakshmanan et al., 2019). It is naturally produced from uric acid by legumes living in symbiosis with nitrogen-fixing bacteria and can be obtained from plants, e.g. S. officinale, Phaseolus vulgaris, and Glycine max (Dresler et al., 2017, 2018). For commercial use, the compound is most often produced in chemical synthesis. However, natural allantoin may slightly differ from the chemically synthesized form in terms of its chemical structure and biological activity (Dinica et al., 2021; Tajima et al., 2004; Xu et al., 2011).
The literature data on the level of allantoin in comfrey root are limited and fragmentary. Therefore, the aim of this study was to compare the concentrations of this compound in selected raw materials of S. officinale roots commercially available on the Polish market. The hypothesis that the dried roots of comfrey available on the Polish market contain significantly different concentrations of allantoin was tested.
. Materials and methods
The comfrey root raw materials (Symphyti Radix) produced by four different Polish companies (marked as A – Dary Natury, B – Farmvit, C – Flos, D – Herbapol) were purchased from stationary pharmacies located in Lublin or through online sales. The samples were taken from three-unit packages of a given product batch. Dry roots were ground in an electric grinder. Then, three aliquots of ground root (200 mg) were prepared as samples from individual companies. The samples were placed in test tubes, mixed with 20 mL of distilled water, and extracted in an ultrasonic bath for 30 min at room temperature. Finally, the extracts were centrifuged at 6,000 rpm (10 min). Measurements of the allantoin concentration were carried out using high-performance liquid chromatography (HPLC). The HPLC chromatograph was equipped with a UV–VIS detector with a photodiode array (VWR Hitachi Chromaster 600, Merck, Germany). The chromatographic separation was performed using a Rezex RPM-Organic Acid Aminex HPX-87H column (300 × 7.8 mm). Sulphuric acid (0.005 N) was used for isocratic elution, the eluent flow was set to 0.6 mL/min, and the column temperature was 40 °C. Detection was made at λ = 195 nm. Allantoin was identified on the basis of its retention time (Figure 1) and comparison of the absorption spectrum with the allantoin standard (Sigma-Aldrich). Quantitative determinations were made on the basis of a calibration curve prepared for the allantoin standard. The pH of the tested extracts (3 g of ground plant material with 150 mL of distilled water) was also determined by a pH-meter (Mettler Toledo, S210).
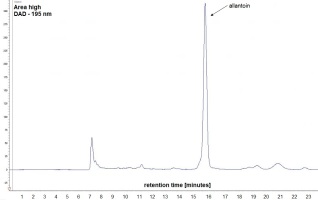
Figure 1
Example of a chromatogram of S. officinale root extract with an allantoin peak identified via the HPLC method (UV detection at 195 nm).
All data sets were tested with one-way analysis of variance (ANOVA), and post hoc analysis using Tukey’s test at a significance level of α = 0.05 was applied.
. Results and discussion
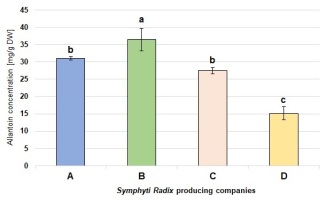
The accumulation of allantoin in comfrey is most probably related to the low ability of this species for enzymatic degradation of ureides (Mazzafera et al., 2008). In our study, the highest concentration of allantoin (36.46 mg/g DW) in the commercially available Symphyti Radix samples was found in the plant material supplied by company B, while the lowest level (15.14 mg/g DW) was recorded in the product from company D. Thus, there was an over two-fold difference between the maximum and the minimum amount. The level of allantoin in the raw materials supplied by companies A and C did not differ significantly and was 31.0 mg/g DW and 27.5 mg/g DW, respectively (Figure 2). In turn, the average value for all the tested samples was 27.5 mg/g DW.
Figure 2
Content of allantoin in comfrey root from four different producers. Mean values (± standard deviation; n = 3) marked with different letters differ statistically significantly at α = 0.05. Abbreviations: companies names A – Dary Natury; B – Farmvit; C – Flos; D – Herbapol.
Limited literature data indicate that the concentrations of this compound may be in the range of 7.0–25.5 mg/g DW (Sousa et al., 1991). Moreover, the allantoin content in comfrey root can vary highly depending on the plant development stage. Therefore, not only the development phase but also the place of cultivation, type of soil, weather conditions, availability of nutrients, conditions of storage and packaging as well as the transport method should be taken into consideration. The highest levels were recorded in the vegetative phase (approx. 20 mg/g) and the lowest content (approx. 0.1 mg/g) was noted during fruit development (Mothes, 1961). According to a recent study conducted by Dresler et al. (2017), the concentration of allantoin in comfrey root was 25.77 mg/g DW, i.e. very close to the mean value obtained in this study. In turn, using the thin-layer chromatography (TLC) technique, Kimel et al. (2020) showed that the content of this compound in raw materials from two different suppliers was 10.6 and 18.4 mg/g DW, which is lower than that obtained in the present study. The differences between these and our results may be a consequence of the use of different analytical methods for the determination of allantoin concentrations (TLC vs. HPLC). A comparison of the content of some secondary metabolites, including allantoin, in 17 species from the Boraginaceae family revealed that comfrey root was rich in allantoin (approx. 26 mg/g DW), but higher concentrations of this compound (approx. 35 mg/g DW) were recorded in the roots of Echium italicum. In this study, the other parameter studied was the pH of root extracts, which was slightly acidic and ranged from 6.22 to 6.43 pH, but these values did not differ significantly between the preparations of raw material supplied by the individual companies. In addition, it was observed that the raw materials provided by the different companies differed in their grinding properties and color.
In summary, our research provided information on the concentrations of allantoin as a bioactive substance in commercial preparations of dried comfrey root and showed that this raw material contained a relatively high level of allantoin. However, attention should be paid to the possibility of fluctuations in the content of this ureide in the raw material due to its diverse origins, time of collection, and methods of storage. Therefore, there is a need to monitor changes in the level of this compound in raw materials which can be an important source of natural allantoin for dermocosmetic and pharmaceutical formulations.


