Introduction
Water deficit stress affects cellular turgescence, opening of stomata, photosynthesis processes, respiration and transpiration, and ultimately reduces crop yield (Bagheri et al., 2020; Darabad et al., 2020; Zhang et al., 2015). Fertilizers are used to supplement soil with essential nutrients to promote plant growth and increase crop productivity under normal and stress conditions (Cordell et al., 2009). Fertilizers usually provide the three major macronutrients (nitrogen, phosphorus, and potassium). Among these nutrients, phosphorus is particularly a critical component because it is limiting, on a large proportion of global arable land, for crop yield, and it is a nonrenewable resource (Aliabadi Farahani et al., 2008). Phosphorus is crucial for plant growth and constitutes approximately 0.2% of plant dry weight; however, it is one of the nutrients most difficult for plants to acquire (Klimek-Kopyra et al., 2016). It is the second most important nutrient after nitrogen; it affects plant growth and yield and plays important roles in physiological processes such as cell division and development, energy transfer (ATP and ADP), signal transduction, macromolecular biosynthesis, photosynthesis, and plant respiration (Das et al., 2015).
Mycorrhizae are mutualistic (symbiotic association) based on the bidirectional nutrient transfer between soil fungi and the roots of vascular plants. While the plant supplies the fungi with sugars produced via photosynthesis, the hyphal network improves the capacity of plants to absorb water and nutrients (Jamiołkowska et al., 2019; Roy-Bolduc & Hijri, 2011). There is an important interaction between plants and mycorrhizal fungi in terms of microbiological and ecological processes (Garg & Chandel, 2010). Arbuscular mycorrhization is a beneficial symbiosis between arbuscular mycorrhizal (AM) fungi and many terrestrial plants (Brundrett & Tedersoo, 2018). This symbiosis promotes the uptake of phosphates and other nutrients by plant hosts at the expense of the carbon they supply to the fungal endosymbiont. It entails the constant exchange of signals between the host and symbiont, which ultimately leads to the formation of nutrient exchange and fungus-accommodation structures called arbuscular within root cortical cells (Choi et al., 2018). In addition, AM fungi have many non-nutritional benefits to plants. AM fungi hyphae enhance water uptake by increasing the absorbing surface of the root system and accessing the smallest soil pores (Smith & Read, 2008). AM symbiosis is known to complement the innate ability of plants to tolerate drought stress and alleviate stress symptoms (Kapoor et al., 2013). Several studies have demonstrated that AM symbiosis can protect host plants against the detrimental effects of drought stress (Augé et al., 2007; Cho et al., 2006; Fan & Liu, 2011; Manoharan et al., 2010; Rodriguez & Redman, 2005). Hazzoumi et al. (2015) showed that the use of mycorrhizal fertilizer under water stress condition resulted in the highest yield of Ocimum gratissimum essential oil. Arango et al. (2012) demonstrated that plants treated with mycorrhiza showed higher fresh weight, dry weight, and larger leaf area than nonmycorrhizal plants. Esetlili et al. (2015) reported that mycorrhiza treatment increased the content of phosphorus, potassium, and calcium in shoots. It also increased the percentage yield of essential oils in peppermint. Among the species studied, treatment with Rhizophagus intraradices resulted in the highest level of phosphorus in the shoots and essential oil yield. The present study aimed to investigate the comparative effects of different species of mycorrhizal fungi and phosphate fertilization on photosynthetic capacity and enzyme activity of peppermint under different irrigation regimes.
Material and Methods
Field Experiment
In order to investigate the effect of AM and P levels on photosynthetic capacity and enzyme activity of peppermint under different water conditions, an experiment was conducted in a split-plot design based on randomized complete block design in three replications at the research station of the Islamic Azad University, Tabriz Branch (46°17′ E, 38°5′ N, 1,360 m above sea level), during the 2017–2018 growing seasons. The pH of soils in this region ranges from alkaline to medium. Soil analysis indicated that soils in this region had a sandy loam texture, with salinity of 1.56 ds m−1 and pH of 7.53 (Table 1).
Table 1
Physical and chemical analysis of experimental soil.
K (mg/L) | K (ppm) | P (ppm) | pH | EC (dS/m) | Clay | Silt | Sand | Organic matter (%) | Organic carbon (%) |
45 | 7.7 | 15.7 | 7.5 | 1.56 | 11 | 13 | 76 | 2.9 | 1.7 |
The treatments included different levels of water deficiency as the main factor, namely: a1: irrigation after 70 mm evaporation from pan of Class A (control), a2: irrigation after 110 mm evaporation from pan of Class A, and a3: irrigation after 150 mm evaporation from pan of Class A; triple super triphosphate fertilizer levels as subfactors, including: without phosphorus fertilization, 25% recommended amount (the recommended amount based on soil test was 90 kg/ha), and 50% recommended amount; and different mycorrhiza species as sub-subfactors, including: nonmycorrhizal inoculation (control), R. intraradices, Funneliformis mosseae, Glomus hoi, and a mixture of the three strains of mycorrhizal fungi. The Class A evaporation pan is circular, 120.7 cm in diameter, and 25 cm deep. It was made of galvanized iron. The pan was mounted on a wooden open frame platform, 15 cm above ground level. It was filled with water to a depth of 5 cm below the rim. The pan was protected by fences to prevent animals from drinking the water in the pan.
In each plot, three rows of cultivation, as listed planted with 45 cm intervals, were created in the eastern and western directions. The spacing of the subplots was based on a one-to-one plot, with the main plots being 1.5 m and spacings of two 2-m blocks. In the early spring, after the preparation of land, different levels of phosphorus were applied based on the planting plan of the experimental plots. After preparing peppermint (Mentha piperita) seedlings in mid-May, they were transported to the main land at the second to third leaf stage. Before planting, different strains of mycorrhiza (9 g from each) prepared from the Water and Soil Institute, Karaj, Iran, were added to each seedling. For mixed fungal species, 3 g was harvested from any three species, making a total of 9 g, and then followed by transplantation. The seedlings were planted 30 cm apart.
Immediately after transplantation, irrigation was carried out every 3 days for establishment of seedlings. Weeding was performed manually at the required times. In order to supply nitrogen fertilizer, urea was applied at a rate of 200 kg per hectare in two steps: half the recommended amount after the establishment of seedlings and another half at the eight–ten leaf stages before water treatments. After the establishment of plants, different levels of water deficiency stress were applied based on irrigation after evaporation from Class A pan. Each irrigation interval was carried out after 70 ± 5, 110 ± 5, and 150 ± 5 mm evaporation from Class A pan. All studied traits were evaluated in the first harvest of the second year of the experiment. The amount of irrigation water for each treatment was calculated based on their total water requirement, randomly sampled from three different parts of each plot and by determining the percentage of soil water content. The amount was calculated at about 24 hr before irrigation. To measure traits, five samples from each plot were harvested. To determine the irrigated water, the following equation was used:
Determination of the Content of Photosynthetic Pigment
To determine the concentration of leaf chlorophyll, 1 g of fresh leaf was cut into small pieces and placed into a specimen bottle containing 10 mL of absolute ethanol and stored in the dark for 2 weeks. One milliliter of the filtered extract was then diluted with 6 mL of absolute ethanol. The absorbance of the chlorophyll solution was measured using a spectrophotometer at 645 and 663 nm. Chlorophyll a (Chl a) and b (Chl b) content and total chlorophyll a + chlorophyll b were estimated using the formula by Porra et al. (2002) as:
Essential Oil Content and Essential Oil Yield
To determine the percentage of essential oil, leaves were dried under shade conditions and powdered using a mill. Essential oils were extracted via water distillation using a Clevenger apparatus. The dry and milled leaves of each treatment were selected for each treatment, which was a sample of 25 g mixed with 500 mL of distilled water and placed inside a balloon. The resulting mixture was heated using a Clevenger apparatus for 3 hr; yellow essential oils were collected and dehumidified using free-water sodium sulfate and diethyl ether, and the percentage of essential oil was calculated. The essential oil yield was obtained by multiplying dry material yield by essential oil percentage.
Stomatal Resistance
Stomatal resistance (SR) was measured using a licor LI-65 diffusion porometer. Two separate selections for SR measurement were taken for each leaf.
Determination of Catalase and Peroxidase Enzyme Activities
To measure enzyme activities, 0.2 g of fresh tissue was used. In order to extract protein, 0.2 g of fresh plant tissue was crushed using liquid nitrogen, and then, 1 mL of buffer Tris-HCl (0.05 M, pH 7.5) was added. The resulting mixture was centrifuged for 20 min (13,000 rpm and 4 °C), and the supernatant used for measurement of enzyme activities (Sudhakar et al., 2001). Catalase (CAT; EC 1.11.1.6) activity was assayed according to the report of Kar and Mishra (1976). Protein extract (60 μL) was added to Tris buffer (50 mM, pH = 7) containing 5 mM H2O2 on an ice bath, and the absorbance curve plotted at a wavelength of 240 nm. Enzyme activity was obtained for OD mg protein min−1 of fresh tissue. Peroxidase (POX; EC 1.11.1.7) activity was measured as proposed by Kar and Mishra (1976): 50 μL protein extract was added to 2.5 mL extraction buffer containing 100 μM Tris buffer (100 mM), hydrogen peroxide (5 mM), and pirogalol (10 mM) in an ice bath, and absorbance changes read at a wavelength of 425 nm. The enzyme activities of CAT and POX were expressed on the basis of mg protein/min.
Statistical Analysis
The experiment was a split-plot in a randomized complete block design with three replicates. After sampling and measuring the traits, statistical analysis of the data was performed based on the statistical model of split plot experimental design using SAS 9.1 software; the means of treatments were compared using Duncan’s multiple-range test at 5% level. Charts were plotted using Excel software.
Results
The effects of experimental factors and their interaction on the traits studied in the first harvest of the second year are presented in Table 2.
Table 2
Analysis of variance of chlorophyll a (Chl a), chlorophyll b (Chl b), stomatal resistance (SR), essential oil percentage (EOP) and essential oil yield (EOY), catalase (CAT), and peroxidase (POX).
Content of Photosynthetic Pigment
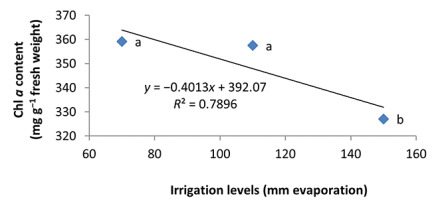
Water deficiency in the second year and first harvest decreased Chl a content in peppermint leaves. By reducing irrigation from a2 to a3, the Chl a content decreased by 8%–9% (Figure 1). Also, there was no significant difference between a1 and a2 treatments.
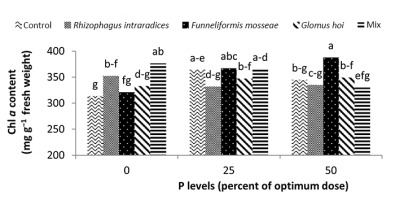
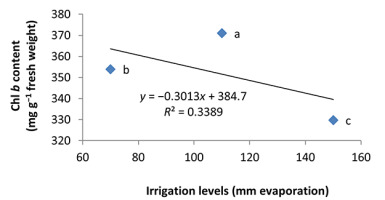
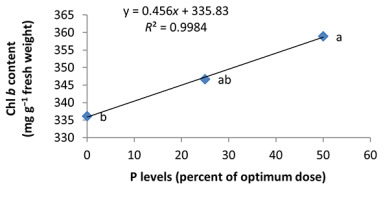
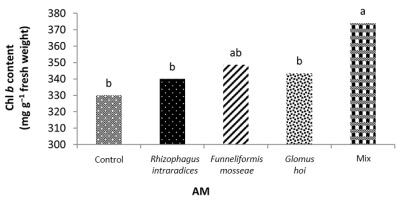
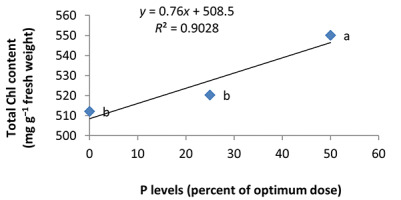
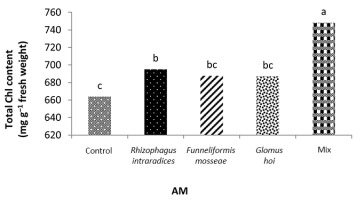
In the nonmycorrhizal inoculation treatments with R. intraradices, F. mosseae, and G. hoi, the increase in the phosphorus fertilizer dose resulted in an increase in the Chl a content by 0.62, 1.3, and 0.33 units, respectively. From the results, the maximum increase, which resulted from the use of phosphorus fertilizer, was obtained in R. intraradices treatment (Figure 2). By reducing irrigation water from a1 to a3, Chl b content reduced by 5.68% and 6.7%; however, a2 and a1 irrigation treatments had no effect on Chl b content (Figure 3). Chl b content reduced by 0.24 units per unit reduction of irrigation water. The treatments of 50% and 25% recommended doses of phosphorus fertilizer increased Chl b content in peppermint leaves by 7.4% and 6.46%, respectively (Figure 4). In the first harvest of the second year, none of the mycorrhizal fertilizer treatments had any effect on the Chl b content of peppermint leaves, and only the combined application of mycorrhizal fertilizers caused a significant increase in Chl b content. In combined mycorrhizal fertilizer application treatment, chlorophyll b content was 364 mg/g weight of fresh leaf and was 9.39% higher than that of nonmycorrhizal inoculation treatment (Figure 5). In this study, the application of only 25% of the optimum dose of phosphorus fertilizer caused a significant increase by 6%–8% in total chlorophyll content (Figure 6). Treatment of 50% optimal dose of phosphorus fertilizer had no significant effect on the total chlorophyll content of peppermint leaves. The application of all three strains of mycorrhizal fungi caused the highest increase in total chlorophyll content. Total chlorophyll content increased by 12.8% compared to that of the control owing to the combined application of all three strains. In the first harvest, R. intraradices treatment caused a significant increase of 4.8% in total chlorophyll content (Figure 7).
Stomatal Resistance
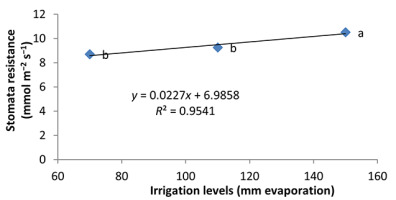
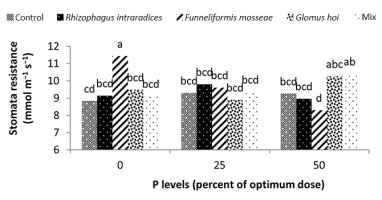
Water deficiency increased stomatal resistance in peppermint leaves. By reducing irrigation water from a1 to a3, stomatal resistance of peppermint leaves increased by 22% and 13.28%, respectively (Figure 8). Stomatal closure is a protective mechanism for reducing the amount of water lost under water deficiency conditions (Pirasteh-Anoosheh et al., 2016). The effect of phosphorus fertilizer was different depending on mycorrhizal species. In this study, F. mosseae treatment and application of 25% recommended P fertilizer caused a significant increase in stomatal resistance and enhanced the trait by 29.5% (Figure 9). Moinuddin et al. (2012) reported that phosphorous fertilizer increases the leaf stomatal conductance of medicinal herbs, probably owing to increase in root growth and access of plants to water.
Percentage and Yield of Essential Oil
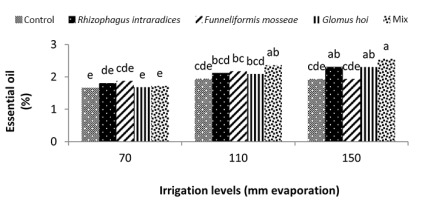
Water deficiency increased the essential oil percentage of peppermint plants. The highest percentage of peppermint essential oil was 34.2% and was obtained from the three species combination treatment under a3 irrigation level. The lowest percentages of essential oil were 1.64% and 1.63%, and were obtained in the nonmycorrhiza and R. intraradices treatments at a1 irrigation level. In G. hoi treatment, water deficiency had no significant effect on the percentage of essential oil of peppermint; however, in other treatments of mycorrhizal fertilizers, water deficiency caused a significant increase in the percentage of essential oil. In F. mosseae, R. intraradices, and a mixture of all mycorrhizal fungi treatments, the reduction of irrigation water from a1 to a3 increased the percentage of peppermint essential oil by 19.5%, 37.1%, and 28.1%, respectively. The highest percentage of essential oil was 2.5% and was observed when a mixture of all three species at a3 irrigation level was used (Figure 10).
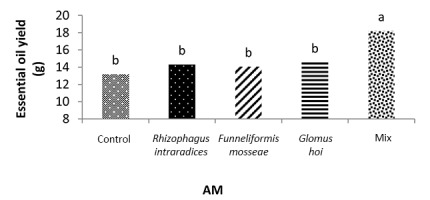
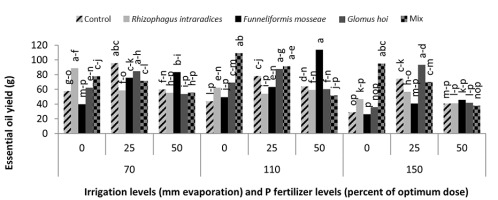
In the mixture of all the three species treatment, the essential oil yield was 18.2 g, which was 38.1% higher than that of nonmycorrhizal inoculation (Figure 11). The highest yield of essential oil was 113 g and was obtained in F. mosseae treatment with 50% recommended dose of phosphate fertilizer and a2 irrigation level. In this study, a3 irrigation with R. intraradices alone and F. mosseae with 25% recommended dose of phosphorus fertilizer caused a 47.2% and 46.9% decrease in the essential oil yield of peppermint, respectively (Figure 12).
Activity of Catalase and Peroxidase
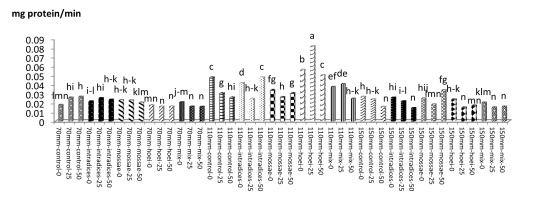
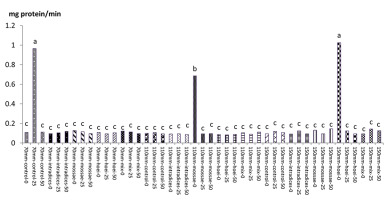
According to mean comparison, the maximum catalase content was obtained from the application of G. hoi mycorrhizal treatment using 25% recommended dose of phosphorus fertilizer and irrigation after 110 mm evaporation with an average of 1.146 mg protein/min, and the minimum catalase content was obtained from the application of the same mycorrhizal fertilizer using 50% of the recommended dose of phosphorus fertilizer and a1 irrigation with an average of 0.073 mg protein per minute (Figure 13). According to results, the application of G. hoi mycorrhizal fertilizer with 25% recommended dose of phosphorus fertilizer and a3 irrigation with an average of 0.082 mg protein per minute had the maximum peroxidase content and the application of R. intraradices mycorrhizal fertilizer using 50% recommended dose of phosphorus fertilizer and a3 irrigation with an average of 0.082 mg protein per minute had the minimum peroxidase content (Figure 14).
Discussion
In this study, mycorrhiza treatments and a1 irrigation had no significant effect on the essential oil percentage of peppermint. In a2 irrigation and mycorrhizal fertilizer treatments alone, there was no significant effect on the essential oil percentage of peppermint; only the use of mixture of mycorrhizal fertilizers increased the essential oil percentage of peppermint significantly. The use of mycorrhizal treatments led to a 21% increase in the essential oil percentage of peppermint. Under critical water deficiency, even the use of mycorrhizal fertilizer alone increased the essential oil percentage of peppermint. In a3 irrigation, R. intraradices and G. hoi treatments and a mixture of all three species increased the essential oil percentage of peppermint by 21%, 21%, and 31.5%, respectively. In nonmycorrhizal inoculation and F. mosseae treatment in the first harvest of the second year, reducing the amount of irrigation water had no effect on the essential oil percentage of peppermint. However, in R. intraradices, G. hoi, and a mixture of all three species treatments, reducing the amount of irrigation water from a1 to a3 decreased the percentage of peppermint essential oil by 27.7%, 43.7%, and 47%, respectively (Figure 10). Zolfaghari et al. (2013) reported an increase in the essential oil percentage of medicinal plants using mycorrhizal fertilizer in Ocimum basilicum. They also showed that the inoculation of Ocimum basilicum with F. mosseae increased the amount of essential oil four folds. Silva et al. (2014) reported that integrated application of mycorrhizal fertilizers had a more positive effect on the essential oil yield of Mentha piperita, owing to extended root colonization by mycorrhiza. In a3 irrigation, using 25% recommended dose of phosphorus fertilizer alone resulted in a 65.9% increase in the essential oil yield of peppermint. In a2 irrigation, treatment of all the three species mixture alone, F. mosseae with 50% recommended dose of phosphorous fertilizer, G. hoi with 25% recommended dose of phosphorous fertilizer, and a mixture of all three species with 25% recommended dose of phosphorous fertilizer caused a significant increase of 153%, 162%, 102%, and 111%, respectively, in the essential oil yield of peppermint. In a3 irrigation, treatment of all three species mixture alone, G. hoi with 25% recommended dose of phosphorous fertilizer, and a mixture of all three species with 25% recommended dose of phosphorous fertilizer produced 239%, 158%, 232%, and 146% increase in the essential oil yield of peppermint, respectively. Arango et al. (2012) showed that R. intraradices and phosphorous were the most effective fertilizer combination for increasing the yield of peppermint. Drought stress often induces cellular and photo-oxidative damage through the accumulation of reactive oxygen species. As a consequence, higher plants evolve cellular responses, such as the upregulation of oxidative stress protectors (Reddy et al., 2004). Antioxidant defense enzymes, such as peroxidases and catalase, are designed to minimize concentrations of hydrogen peroxide with oxidative effects in cells (Apel & Hirt, 2004). Ruiz-Lozano (1996) reported that AM symbiosis might increase the drought resistance of higher plants by promoting antioxidant enzymes. They proposed that higher antioxidant enzyme activity in mycorrhizal plants helps in the rapid and efficient removal of excess ROS. However, reports on the influence of AM symbioses on antioxidant system under stress conditions are not adequate (Evelin & Kapoor, 2014).
Conclusion
Applying a mixture of mycorrhizal species and the application of 50% phosphorus fertilizer can improve the essential oil yield and physiological characteristics of peppermint. Based on the results of this study, in the first harvest of the second year, the highest percentage of essential oil was 2.5%, and was observed when a mixture of all three species at a3 irrigation. Moreover, in the treatment of all the three species mixture, the essential oil yield was 18.2 g, which was 38.1% higher than that of the nonmycorrhizal fertilizer.
Handling Editor
Barbara Hawrylak-Nowak; University of Life Sciences in Lublin, Poland; https://orcid.org/0000-0002-2935-1491