. Introduction
For the past three decades, micropropagation has played a lead role in the rapid multiplication of disease-free and high-grade plant materials (Ziv, 1991). The application of this technology to small-fruit species and vegetative rootstocks is the most successful example for the commercial application of in vitro culture for fruit crops (Litz, 2005). In vitro grown plants are maintained on nutrient medium supplemented with carbohydrates, which results in heterotrophic or mixotrophic growth (Desjardins, 1995; Grout & Donkin, 1987; Kozai et al., 1990). During acclimatization to ex vitro conditions, plants are required to convert to autotrophic carbon assimilation (Pospíšilová et al., 1996; Van Huylenbroeck & De Riek, 1995); therefore, rooting and acclimatization are key steps in micropropagation. However, the widespread use of micropropagated transplants is still limited by the high production costs in relation to low growth rates, a significant loss of plants in vitro due to microbial contamination, poor rooting, low survival percentage at the ex vitro acclimatization stage, and high labor costs. Pear (Pyrus) cultivars and species are often recalcitrant to tissue culture manipulations, although propagation protocols for different pear genotypes have been reported (Bell & Reed, 2002; Chevreau et al., 1992; Dimitrova et al., 2016; Nacheva et al., 2009; Reed et al., 2013). Similar to other woody species of the Rosaceae family, rooting in vitro has proven to be difficult and highly genotype-dependent (Nemeth, 1986; Reed, 1995). There is currently no consensus as to the best method for rooting Pyrus microcuttings among the many approaches that have been attempted (Barros et al., 2005; Liu et al., 2004; Luo et al., 2006; Reed, 1995). Pears and other species require auxin treatment, generally with indolyl-butyric acid (IBA) and 1-naphthaleneacetic acid (NAA) (Klimek-Kopyra et al., 2019; Monder 2019; Zhao & Hasenstein, 2010). Recently, reports of simultaneous rooting and acclimatization have been reported in the scientific literature. According to some authors, plantlets developed from ex vitro rooting have potential advantages in comparison to those developed from in vitro rooting, such as better root systems, higher root numbers, easier acclimatization, and greater survival percentage (Benmahioul et al., 2012; Yan et al., 2010). Ex vitro rooting is not only advantageous for the acclimatization of plantlets, but also for the reduction of labor and costs of micropropagation (Annapurna & Rathore, 2010; Benmahioul et al., 2012; Huang et al., 2011; Phulwaria, Rai, Harish, et al., 2012; Phulwaria et al., 2011; Phulwaria, Ram, Harish, et al., 2012). Successful ex vitro rooting has been applied in the micropropagation of various plant species, including blueberries (Vaccinium corymbosum L. and Vaccinium angustifolium × corymbosum) (Isutsa & Pritts, 1994), raspberries (Rubus idaeus L.) (Lebedev et al., 2019), pistacia (Pistacia vera L.) (Benmahioul et al., 2012), and other ornamental and medicinal species (Annapurna & Rathore, 2010; Huang et al., 2011; Phulwaria, Rai, Harish, et al., 2012; Phulwaria et al., 2011; Phulwaria, Ram, Harish, et al., 2012; Podwyszynska & Gabryszewska, 2003; Yan et al., 2010). Reports on the simultaneous rooting and acclimatization of Pyrus species on different substrates are scarce and show little success. The first experiments for ex vitro rooting (treatment with Dip’N Grow) of three pear rootstocks (Pyrus calleryana Dcne ‘OPR 157’, P. betulifolia Bunge ‘OPR 260,’ and P. communis L. ‘OH × F230’) were unsuccessful (Yeo & Reed, 1995). The aim of this study was to improve the micropropagation protocol by ex vitro rooting of pear plantlets using a new generation plant biostimulator of natural origin, Charkor (TU U 24.2-03563790-041-2001; Agrobiotech, Ukraine) as an alternative to synthetically produced PGR. Charkor is a water-alcohol extract of metabolism products (amino acids, fatty acids, sugars, macro- and microelements, and analogs of phytohormones) of in vitro-cultivated symbiotic fungus-endophytes of ginseng roots. According to Ponomarenko and Anishin (2010), Charkor is more effective than IAA and IBA in rooting the cuttings of a number of ornamental trees and shrubs. Our preliminary experiments on rooting in vitro-raised microshoots of magnolia (Gercheva et al., 2015) and pistacia (Nacheva et al., 2019) with nutrient media enriched with Charkor were very successful. In addition, when rooting pear in vitro, this biostimulator showed a prolonged positive effect on the growth of young plants during the acclimatization stage (Dimitrova et al., 2019).
. Material and Methods
Plant Material
The experiments were conducted with plants of pear rootstock ‘Old Home’ × ‘Farmingdale’ OHF 333 (P. communis L.), which are preferred because of their high yield, good compatibility with most pear varieties, and resistance to fire blight (Van der Zwet & Beer, 1995; Wertheim, 2002).
Growing Conditions
The in vitro culture was maintained as a 3-week subculture on a modified MS medium (Murashige & Skoog, 1962) as described previously (Dimitrova et al., 2016; Nacheva et al., 2009) with some modifications. Briefly, the concentration of NH4NO3 was reduced to half strength, CaCl2 was replaced with 1,000 mg L−1 Ca(NO3)2, and the medium was supplemented with 2.5 µM 6-benzylaminopurine (BAP), 0.005 µM IBA, 30 g L−1 sucrose, 6.5 g L−1 Phyto agar (Duchefa Biochemie, the Netherlands), at a pH of 5.6. Cultures were incubated in a growth chamber at a temperature of 22 ± 2 °C and photoperiod 16/8 hr supplied by cool-white fluorescent lamps (OSRAM 40 W; 40 µmol m−2 s−1 photosynthetic photon flux density – PPFD). For the purpose of the experiment, the shootlets obtained during multiplication were elongated on hormone-free nutrient medium for 10 days. Apical microcuttings (20 mm in length) were subjected to four different treatments for ex vitro rooting: 1 g L−1 powdered NAA, 0.5 mL L−1 Charkor for 3 or 6 hr, and the same concentration of Charkor in powdered form. Microshoots treated with sterile distilled water containing no additional hormonal treatments served as controls. Multicell bedding plant trays (105 cells) filled with peat:perlite in a 1:1 (v/v) ratio were used. Cultures were kept in a growth chamber under a 16-hr photoperiod (fluorescent tubes OSRAM 40 W; 60 µmol m−2 s−1 PPED) and greater than 96% air humidity for 2 weeks, which was then gradually reduced to 60%. Data on final acclimatization rate (survival rate; %), mean number of roots per plant, stem and root length, and mean number of leaves per plant were collected 90 days after transplanting to ex vitro conditions.
Statistical Analysis
All treatments were performed in duplicate with groups of 28 plants and three replicates for each treatment. The experimental results were analyzed with analysis of variance (p ≤ 0.05), and Duncan’s multiple range test was used for mean comparison using SPSS statistical software (version 13 for Windows).
. Results
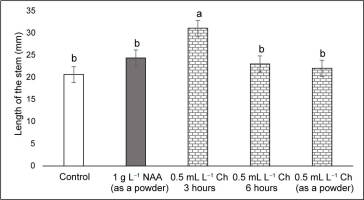
At the end of the acclimatization period (90 days), the highest survival rate of 86% was observed in the treatment with Charkor for 6 hr, and the lowest was reported at 31% for the control (Figure 1, Figure 2). In all treatments with NAA or Charkor, a high survival rate of over 67% was achieved. The length of the plant stem from the different treatments did not differ significantly, except for Treatment 3 (Charkor for 3 hr), in which the stems were longer (Figure 3).
Figure 1
Appearance of pear rootstock OHF 333 (Pyrus communis L. ‘Old Home’ × ‘Farmingdale’) at the end of acclimatization (ninetieth day).

Figure 2
Survival rate (%) of pear rootstock OHF 333 (Pyrus communis L. ‘Old Home’ × ‘Farmingdale’) plants 90 days after transfer to ex vitro conditions. Treatments for ex vitro rooting: 1 – control (without growth regulators); 2 – with 1 g L−1 NAA (as a powder); 3 – 0.5 mL L−1 Charkor for 3 hours; 4 – 0.5 mL L−1 Charkor for 6 hours; 5 – 0.5 mL L−1 Charkor prepared as a powder. Different letters indicate significant difference between values at (p < 0.05).
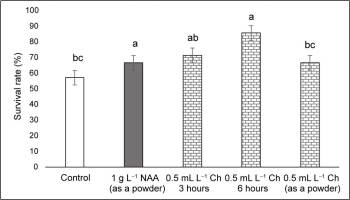
Figure 3
Length of the stem (mm) of pear rootstock OHF 333 (Pyrus communis L. ‘Old Home’ × ‘Farmingdale’) plants 90 days after transfer to ex vitro conditions. Treatments for ex vitro rooting: 1 g L−1 NAA (as a powder), and 0.5 mL L−1 Charkor for 3 hours, 6 hours, or prepared as a powder. Different letters indicate significant difference between values at (p < 0.05).
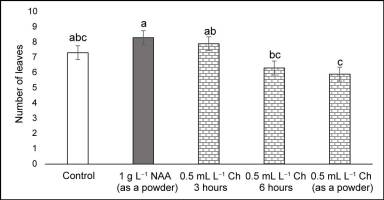
Plants treated with NAA in the stem base had the greatest number of roots (>5), followed by those treated with Charkor for 3 hr (Figure 4). No significant difference in root length was observed between the individual treatments (Figure 5). Regarding the number of roots, no significant difference between the individual treatments was reported, except for the Charkor treatment in the powdered form, which revealed a greater number. However, as shown in Figure 1, the roots of the plants in the control treatment were long, but without the development of lateral roots. Treatment of the plant base with NAA or Charkor stimulated the development of a more compact root system with numerous small lateral roots, which favored the uptake of more water and nutrients from the substrate. The highest number of leaves was observed in NAA-treated plants (Figure 6), followed by Charkor-treated plants for 3 hr and control plants. The lowest leaf number was observed in plants in which Charkor was applied as a powder.
Figure 4
Number of roots of pear rootstock OHF 333 (Pyrus communis L. ‘Old Home’ × ‘Farmingdale’) plants 90 days after transfer to ex vitro conditions. Treatments for ex vitro rooting: 1 g L−1 NAA (as a powder), and 0.5 mL L−1 Charkor for 3 hours, 6 hours, or prepared as a powder. Different letters indicate significant difference between values at (p < 0.05).
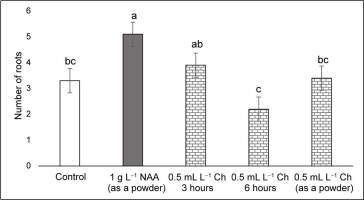
Figure 5
Length of the roots (mm) of pear rootstock OHF 333 (Pyrus communis L. ‘Old Home’ × ‘Farmingdale’) plants 90 days after transfer to ex vitro conditions. Treatments for ex vitro rooting: 1 g L−1 NAA (as a powder), and 0.5 mL L−1 Charkor for 3 hours, 6 hours, or prepared as a powder. Different letters indicate significant difference between values at (p < 0.05).
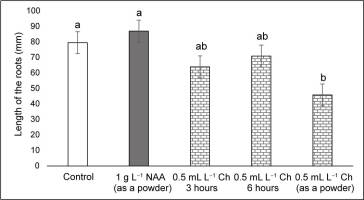
Figure 6
Number of leaves of pear rootstock OHF 333 (Pyrus communis L. ‘Old Home’ × ‘Farmingdale’) plants 90 days after transfer to ex vitro conditions. Treatments for ex vitro rooting: 1 g L−1 NAA (as powder), and 0.5 mL L−1 Charkor for 3 hours, 6 hours, or prepared as a powder. Different letters indicate significant difference between values at (p < 0.05).
. Discussion
Biometric parameters (number of roots per plant, root length, and number of leaves per plant) were greater in plants treated with NAA, although differences in stem and root length and number of leaves were not statistically proven (Figure 4–Figure 6). It is noteworthy that treatment with Charkor for 6 hr increased the rate of plant survival compared to the shorter treatment for 3 hr, but decreased the number of roots (Figure 2, Figure 5). Some authors have stated that optimal auxin concentrations for root initiation can inhibit root elongation. Both rooting and acclimatization are highly dependent on genotype. According to Reed (1995), 72% of in vitro-rooted shoots from 28 genotypes survived acclimatization and developed in the greenhouse, with some genotypes reaching 100% acclimation survival, while others succumbed, such as P. cordata Desv. and P. ussuriensis. According to other authors, the survival rates reported for transplanted, in vitro-grown pear shoots vary from 30% to 90% for P. communis cultivars (Marino, 1988; Rodriguez et al., 1991; Viseur, 1987), and 50% to 73% for P. pyrifolia seedling-derived shoots (Bhojwani et al., 1984). The survival percentage, similar to rooting numbers, varied among ploidy levels, with diploid being the highest (90%), followed by triploid (70%), tetraploid (60%), and lastly, mixploid (45%) (Sun et al., 2009). Rooting and survival of microcuttings can also be limited by their ability to regulate water loss through leaves (Brainerd & Fuchigami, 1982; Desjardins, 1995; Grout & Donkin, 1987). Zhu et al. (2003) noted only 5% rooting and successful acclimatization of pear rootstock BP10030 (P. communis L.) under nonsterile (ex vitro) conditions. They achieved rooting percentages between 71% and 100% only with transformed rolB gene transgenic clones. Moon and Lee (2008) achieved up to 50% ex vitro rooting of the shoot cuttings of P. ussuriensis Maxim. using 100 mg L−1 IBA. Aygun and Dumanoglu (2015) investigated ex vitro rooting of in vitro-raised microcuttings of P. elaeagrifolia Pallas by treating the plant base with different concentrations of IBA (0, 10, 20, 30, and 40 mM). They obtained between 0% and 55% rooting, with the highest percentage (55%) and the highest number of roots per plant (1.8) reported at the lowest tested concentration of 10 mM IBA. In many genera, rooting of micropropagated shoots can be accomplished by removing cytokinins from the growth medium (Arrillaga et al., 1991; Kerns & Meyer, 1986). In pear and other woody plants, this approach is less successful than auxin treatment (Bhojwani et al., 1984; Dolcet-Sanjuan et al., 1990; Manzanera & Pardos, 1990). Poor rooting on hormone-free medium was confirmed in only 9% of genotypes rooted without auxin treatment (Reed, 1995). IBA has been a successful rooting treatment for some pear genotypes, although it has been associated with increased callus production. According to Dolcet-Sanjuan et al. (1990), some pear genotypes responded better to NAA than to IBA, but for others, NAA and IBA led to similar results. Due to the variable rooting response in the Pyrus genus, different treatments are required for each genotype. In our previous experiment on in vitro rooting of pear rootstock OHF 333, Charkor (0.5 ml L−1) had a long-term positive effect on the growth of young plants at the acclimatization stage (Dimitrova et al., 2019). Our previous experiment revealed that enriching the nutrient medium with 1 mL L−1 Charkor led to a significant stimulation of magnolia rooting (100%), compared with the control (33% for Magnolia ×soulangiana Soul.-Bod. and 0% for Magnolia grandiflora L.), accompanied by a 3–5 times greater number of roots per plant (Gercheva et al., 2015). We achieved a significant increase in rooting of micropropagated Pistacia terebinthus L. plants on agar media, supplemented with 1 mL L−1 Charkor, from 4% in the control plants to 33% in Charkor-treated plants (Nacheva et al., 2019). Combining Charkor (1 mL L−1) with low concentrations of auxins (10 µM IBA and 0.054 µM NAA), the rooting percentage of Pistacia plantlets increased to 76%. The results of the present study with pear rootstock OHF 333 confirmed the stimulating influence of Charkor on root formation of micropropagated Magnolia and Pistacia, as previously established (Gercheva et al., 2015; Nacheva et al., 2019). The findings revealed here indicate that the microcuttings from the pear rootstock OHF 333 can be successfully rooted in nonsterile ex vitro conditions simultaneously with their acclimatization. The application of biostimulants such as NAA and Charkor could improve this process, with over 85% of plants achieving successful acclimatization. Simultaneous rooting and acclimatization of in vitro obtained microshoots improves micropropagation efficiency due to time and cost reduction and a simplification of the process by the removal of a step (in vitro rooting on agar medium). It also eliminates the risk of root damage during transplantation of rooted plants. Recently, this technology has been successfully applied to a number of herbaceous and woody species. For example, ex vitro rooting reduced the cost of micropropagated tea Camellia sinensis (L.) O. Kuntz plants by 71% compared with conventional in vitro rooting (Ranaweera et al., 2013). According to Sisunandar et al. (2018), the use of this technology reduced the time of in vitro culture for Kopyor coconut plants from 10 to 4 months. The rooting and transplant survival rates were also found to be higher in the ex vitro method in Siratia grosvenorii (Yan et al., 2010). Most importantly, the plantlets developed through the ex vitro rooting method, similar to the natural root system of S. grosvenorii, had lateral roots without any callus at the base of microcuttings, making the ex vitro rooting method more suitable than the in vitro development of roots. Lebedev et al. (2019) reported that ex vitro rooting of raspberry could reduce the cost and labor intensity of plant micropropagation. Unrooted microshoots of the primocane-fruiting raspberry cultivar ‘Atlant’ were simultaneously rooted and acclimatized in the greenhouse with a survival rate of up to 97.2%. One-step ex vitro rooting, comprising both rooting and hardening, reduces labor, time, and cost of micropropagated plantlets, and could aid in the acclimatization of plantlets. Studies by various authors have confirmed the successful rooting of microshoots of many plant species under ex vitro conditions and their survival when planted in the field (Annapurna & Rathore, 2010; Benmahioul et al., 2012; Huang et al., 2011; Phulwaria, Rai, Harish, et al., 2012). Plantlets developed from ex vitro rooting have potential advantages in comparison to plantlets developed from in vitro rooting, such as better root systems, higher root numbers, ease of acclimatization, and higher survival percentages. Moreover, plantlets rooted on the greenhouse bench under mist can tolerate transplant stress better than plantlets rooted in vitro (Benmahioul et al., 2012; Yan et al., 2010).
. Conclusion
In vitro-raised microcuttings of pear rootstock OHF 333 (P. communis L.) could be successfully rooted in nonsterile conditions, simultaneously with ex vitro acclimatization in a closed environment. The plants then develop and become ready to be planted in a greenhouse. The application of biostimulants such as NAA and Charkor could improve this process, with over 85% of pear plants achieving successful acclimatization. The greatest mean number of roots per plant, root length, and number of leaves was achieved in the variant treated with 1 g L−1 of powdered NAA. The natural biostimulator Charkor could have potential as an alternative to synthetic growth regulators in the search for innovative products for sustainable agriculture use, and Charkor treatment could also be recommended for ex vitro rooting of other recalcitrant genotypes.


