Abbreviations
FBA: fructaldolase;
CBC: Calvin-Benson cycle;
FBA/P: D-fructose-1,6-bisphosphate;
DHAP: dihydroxyacetone phosphate;
G3P: D-glyceraldehyde-3-phosphate;
EMP: Embden-Meyerhof-Parnas;
TCA: tricarboxylic acid;
TIM: triosephosphate isomerase;
RuBP: ribulose 1,5-bisphosphate;
3PGA: 3-phosphoglycerate;
1,3-PGA: 1,3-bisphosphoglycerate;
GAP: glyceraldehyde 3-phosphate;
F6P: fructose 6-phosphate;
E4P: erythrose 4-phosphate;
SBP: sedoheptulose 1,7-bisphosphate;
S7P: sedoheptulose 7-phosphate;
Xu5P: xylulose 5-phosphate;
R5P: ribose 5-phosphate;
Ru5P: ribulose 5-phosphate;
G6P: glucose-6-phosphate;
G1P: glucose 1-phosphate;
ADPG: ADP-glucose;
UDPG: UDP-glucose;
FBA/P: FBP aldolase-phosphatase bifunctional enzyme;
AMPK: adenosine monophosphate-activated protein kinase;
ROS: reactive oxygen species;
RNS: reactive nitrogen species;
TBP: D-tagatose 1,6-bisphosphate
. Introduction
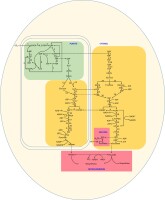
Phosphorylated ketose and aldose are essential metabolites in cellular pathways across all species (Tittmann, 2014). Aldolase enzymes catalyze retroaldol and aldol reactions, resulting in C–C bond cleavage or formation (Galkin et al., 2009). Fructose-1,6-bisphosphate aldolase (E.C. 4.1.2.13), or fructaldolase (FBA), is a well-characterized aldolase that splits the carbon skeleton of ketose phosphates (Galkin et al., 2009). The function of this enzyme is amphibolic, being involved in both catabolic (i.e., glycolysis) and anabolic (i.e., gluconeogenesis and Calvin–Benson cycle [CBC]) pathways. Specifically, FBA catalyzes the reversible conversion of hexoketose D-fructose-1,6-bisphosphate (FBP) to ketose dihydroxyacetone phosphate (DHAP) and aldose D-glyceraldehyde-3-phosphate (G3P) in both the gluconeogenic/glycolytic pathway (the Embden–Meyerhof–Parnas [EMP] pathway) and the CBC. Together with the reductive tricarboxylic acid (TCA) cycle, the reductive pentose phosphate cycle, and the Entner-Doudoroff pathway, the EMP pathway is considered a central metabolic pathway whose enzymes are widely distributed (Ronimus & Morgan, 2003). Research suggests that the EMP pathway likely originated as an anabolic pathway and gained a role in catabolism later (Romano & Conway, 1996; Ronimus & Morgan, 2003). Because of its role in carbohydrate biosynthesis, the CBC pathway is crucial to plant metabolism, growth, and survival (Figure 1).
Figure 1
Calvin cycle and Embden–Meyerhof–Parnas pathways in different cellular compartments.
RuBP: ribulose 1,5-bisphosphate; 3PGA: 3-phosphoglycerate; 1,3-PGA: 1,3-bisphosphoglycerate; DHAP: dihydroxyacetone phosphate; GAP: glyceraldehyde 3-phosphate; FBP: fructose 1,6-bisphosphate; F6P: fructose 6-phosphate; E4P: erythrose 4-phosphate; SBP: sedoheptulose 1,7-bisphosphate; S7P: sedoheptulose 7-phosphate; Xu5P: xylulose 5-phosphate; R5P: ribose 5-phosphate; Ru5P: ribulose 5-phosphate; G6P: glucose-6-phosphate; G1P: glucose 1-phosphate; ADPG: ADP-glucose; UDPG: UDP-glucose;
① RuBP carboxylase/oxygenase (Rubisco); ② 3PGA kinase (PGK); ③ GAP dehydrogenase (GAPDH); ④ triose phosphate isomerase; ⑤ FBP aldolase (aldolase); ⑥ fructose 1,6-bisphosphatase (FBPase); ⑦ transketolase (TK); ⑧ sedoheptulose 1,7-bisphosphatase (SBPase); ⑨ ribulose phosphate 3-epimerase; ⑩ ribose 5-phosphate isomerase; ⑪ phosphoribulokinase (PRK); ⑫ hexokinase; ⑬ phosphoglucose isomerase; ⑭ phosphofructokinase; ⑮ glyceraldehyde-3-phosphate dehydrogenase; ⑯ phosphoglyceric kinase; ⑰ phosphoglyceromutase; ⑱ enolase; ⑲ pyruvate kinase; ⑳ alpha-and β-amylase; ㉑ glucophosphomutase; ㉒ phosphorylase; ㉓ invertase; ㉔ sucrose synthase; ㉕ uridine diphosphate glucose pyrophosphorylase.
Based on their distinct evolutionary history and reaction mechanisms, two unrelated classes of FBAs have been identified: class I (FBA I) and class II (FBA II) (Alefounder et al., 1989; Kobes et al., 1969; Lebherz & Rutter, 1969). FBAs of both classes exhibit the same (β/α)8-triosephosphate isomerase-barrel fold and catalyze the same reactions (Lorentzen et al., 2004), suggesting that they share a common origin (i.e., the ancestral β/α barrel) (Nagano et al., 2002; Sanchez et al., 2002). However, the classes differ significantly from one another in amino acid sequence homology (Imanaka et al., 2002; Marsh & Lebherz, 1992), conserved catalytic residues (Blom et al., 1996), active site locations within the TIM barrel, and reaction mechanisms (Nagano et al., 2002; Sanchez et al., 2002), suggesting independent evolution and/or large insertions or deletions. Studies in both artificially and naturally derived aldolase mutants indicate that changes in protein structure or stability can alter enzymatic activity (Rellos et al., 2000), even plant death in mutant Arabidopsis (Carrera et al., 2021).
. Structure and characteristics of FBA I
The distribution of FBA during evolution is complex and contradictory. FBA I members are found in higher organisms, including animals and vascular plants, green algae, ferns, and mosses. However, FBA I members are also found in archaea (Lorentzen et al., 2004; Morse & Horecker, 1968; Rutter, 1964; Sauve & Sygusch, 2001; Thomson et al., 1998), suggesting a conserved evolutionary history between the two FBA classes (Siebers et al., 2001). The crystal structure of FBA I indicates that the enzyme of 160 kDa is homo-tetrameric and that each subunit contains an independent, active site (Plaumann et al., 1997; Siebers et al., 2001; Sygusch et al., 1987; Thomson et al., 1998). The active site of FBA I is a lysine residue, which can form a Schiff-base intermediate with the substrate (Rutter, 1964). The activity of FBA I can be inhibited by borohydride (NaBH4) (Marsh & Lebherz, 1992; Siebers et al., 2001).
. A novel FBA isoform
In 1998, a novel FBA was identified in Escherichia coli (Thomson et al., 1998). According to the Schiff-base mechanism, the novel FBA behaves like FBA I, although the FBA from E. coli shares very low sequence similarity with classical class I FBAs. Because the FBA from E. coli shares somewhat higher homology (13–20%) with plant dehydrins and is dehydration-responsive, it was originally mis-annotated as dehydrin A (DhnA, dhnA) (Thomson et al., 1998). However, when the gene product was biochemically characterized, it was found to share all the properties of classical class I FBAs (Thomson et al., 1998). Orthologous sequences have been identified in other microbial genomes, including from the archaea Archaeoglobus, Pyrococcus horikoshii, Methanococcus jannaschii, Methanobacterium thermoautotrophicum, and Aeropyrum pernix, and from the bacterium Aquifex aeolicus, a Gram-negative bacterium that belongs to the deeply rooted phylum Aquificae (Dandekar et al., 1999; Galperin et al., 2000; Guiral & Giudici-Orticoni, 2021). In some archaea, both FBA I and FBA II activities have been demonstrated (Altekar & Dhar, 1988; D’Souza & Altekar, 1982; Krishnan & Altekar, 1991; Yu et al., 1994), although no genes encoding classical FBA I or FBA II have been identified in the fully-sequenced archaeal genomes (Lorentzen et al., 2004). When the dhnA gene homologs from the hyperthermophilic archaeon Thermoproteus tenax and the archaeon Pyrococcus furiosus were expressed in E. coli, the recombinant FBA exhibited catabolic substrate specificity for FBP, as well as metal-independent Class I FBA activity by way of a Schiff-base mechanism (Siebers et al., 2001).
Phylogenetic analyses of the T. tenax- and P. furiosus-derived enzymes strongly suggest that this novel enzyme family represents a typical archaeal FBA (Siebers et al., 2001). In Pyrobaculum aerophilum, which apparently possesses an otherwise complete glycolytic pathway, no aldolase genes have been identified. However, this may be because the archaeal FBA represents a new family of aldolases, which is significantly divergent from the classical FBAs and would be difficult to identify by conventional sequence-comparison techniques. Some organisms do possess genes encoding FBA I or FBA II, but due to low sequence similarity with known gene families, these novel FBAs have been designated archaeal Class I aldolases (FBA IA) (Siebers et al., 2001). On the one hand, although FBA IA shares low sequence similarity (20%) with classical FBA I, it is considered homologous but evolutionarily distant due to the presence of a Schiff-base-forming lysine and a common phosphate-binding site (Siebers et al., 2001). On the other hand, the highly divergent sequence signatures suggest a distant relationship between FBA IA and FBA II (Galperin et al., 2000). The highly conserved active site shared between FBA I and FBA IA suggests that they share a common ancestor; however, no eukaryotic FBA IA homologues have been discovered to date (Lorentzen et al., 2004).
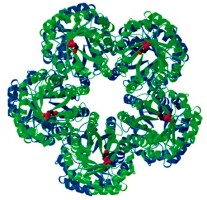
The three-dimensional structure of FBA IA has been characterized as either a homo-octamer, a decamer, or even higher oligomers (Krishnan & Altekar, 1991; Siebers et al., 2001; Thomson et al., 1998). For example, the T. tenax-derived FBA IA (Tt-FBA IA) forms a homo-decamer consisting of two identical pentamer rings, the latter of which is a highly stable structure for its complete closed loop, and the buried surface area in pentamer formation is as high as 26% of the total accessible surface area of the monomer (Lorentzen et al., 2004) (Figure 2). The two pentamers assemble with the barrel N-termini facing each other and the C-terminal active sites facing the surface. Furthermore, the pentamer interface is highly hydrophobic, which likely ensures thermostability.
Figure 2
Ribbon representation of the crystal structure of the doughnut-shaped decameric FBP aldolase from T. tenax viewed along the 5-fold axis of the pentamers (Lorentzen et al., 2004). The two pentamers are colored blue and green, respectively, and the substrate DHAP is shown in a spacefill in red representation. Each monomer has one substrate molecule bound at the active site located in the C-terminal of the TIM barrel (for clarity, the substrates are only shown for one of the two pentamers).
In E. coli, a different aldolase, L-fuculose-1-phosphate aldolase (fucA), possesses as much as 4% fructose-1,6-bis-phosphate aldolase activity (Dreyer & Schulz, 1993). However, it has been experimentally challenging to detect FBA activity in archaea; where activity has been detected, the activity was measured only in the direction of FBP formation and failed in the reverse direction (Jahn et al., 2007). This discrepancy is baffling because the reaction catalyzed by FBA should be reversible. Many archaea, such as Thermococcus kodakarensis and Sulfolobus tokodaii (Nishimasu et al., 2004), possess a bifunctional FBP aldolase-phosphatase (FBA/P), which exhibits both FBP aldolase and FBP phosphatase activity (Say & Fuchs, 2010). FBA/P mediates the aldol condensation of heat-labile dihydroxyacetonephosphate (DHAP) and glyceraldehyde-3-phosphate (GAP) to FBP, as well as the subsequent irreversible hydrolysis of the product to yield stable fructose-6-phosphate (F6P) and inorganic phosphate by remodeling its active site according to the respective catalytic requirements (Du et al., 2011; Siebers et al., 2001).
. Structure and characteristics of FBA II
Class II FBAs (FBA II) are primarily found in bacteria and fungi (Rutter et al., 1968). The class II FBAs typically exhibit molecular weights of approximately 80 kDa, and bacterial FBA IIs exhibit higher crystal structure variability than those from fungi (Plaumann et al., 1997; Siebers et al., 2001; Thomson et al., 1998). Additionally, contrary to FBA of class I, bacterial FBA IIs have been discovered to contain subunits ranging from 27–40 kDa in mass (Plaumann et al., 1997; Thomson et al., 1998). Furthermore, class II FBAs do not share a common active site with class I FBAs.
Studies on organized structures isolated from prokaryotic and eukaryotic organisms demonstrate that, in vivo, enzymes always bind to other proteins in a highly specific manner to form more complex structures, as well as interact with other cellular components, such as membranes (Graciet et al., 2004). These highly complex structures participate in an array of cellular functions (Gavin et al., 2002; Kumar & Snyder, 2002). FBA II has been found to be localized to the cell wall, cell membrane, and cell surface. Whereas FBA I is always located in chloroplast or cytoplasm (de Paz et al., 2007). Furthermore, class II FBAs require divalent metal cations at their active sites in order to stabilize the carbanion intermediate (Hall et al., 1999; Siebers et al., 2001; Zgiby et al., 2000, 2002) and their activity can be competitively inhibited by EDTA, a metal ion chelating agent. Specifically, most class II aldolases require Zn2+ for activation. However, the Deinococcus radiodurans-derived aldolase preferentially utilizes Mn2+, rather than Zn2+, as a cofactor (Zhang et al., 2006).
Both classes of FBAs have been found in Mycobacterium tuberculosis (Bai et al., 1974), E. coli (Stribling & Perham, 1973), and the thermotolerant Gram-positive methylotroph Bacillus methanolicus (Stolzenberger et al., 2013). In B. methanolicus, two distinct class II enzymes were found to be encoded on the nucleoid and on a plasmid, respectively (Stolzenberger et al., 2013). Based on their crystal structures, class II FBAs can be classified as either type A or type B (Plaumann et al., 1997). Type A enzymes are dimeric and are primarily involved in glycolysis and gluconeogenesis, while type B enzymes can be either dimeric, tetrameric, or octameric (Nakahara et al., 2003; Sauve & Sygusch, 2001).
. FBA in pathogenic microorganisms
Most FBAs isolated from pathogenic microorganisms belong to class II. Studies suggest that pathogen-derived FBAs take on different roles and that their function is positively correlated with the age and identity of the host (Ling et al., 2004). Pathogen-derived FBAs may perform two or more biochemical functions utilizing a single polypeptide chain by involving in an array of intracellular biochemical functions (Henderson & Martin, 2011; Sherawat et al., 2008) in several bacterial species that cause illness (Shams et al., 2014). Knock-down of these aldolases resulted in cell death (Lew & Tolan, 2012) and adenosine monophosphate-activated protein kinase (AMPK) activation, even under high glucose conditions (Zhang et al., 2017).
Pathogen-derived FBAs have been reported to exhibit non-glycolytic functions. In Francisella tularensis, an intracellular pathogen responsible for tularemia infects a variety of cell types, FBA has been reported to function as a transcriptional regulator, affecting the pathogenicity of the organism (Ziveri et al., 2017). Furthermore, F. tularensis-derived FBA (Ft-FBA) is responsive to oxidative stress and appears to lie at the intersection of carbon metabolism and regulation of host redox homeostasis, causing the generation of reactive oxygen and nitrogen species (ROS/RNS) in infected phagocytic cells. In higher plants, ROS is usually accumulated under low-temperature conditions and results in the inhibition of Calvin cycle enzymes. The antioxidant defense system is always there to counter reactive oxygen/nitrogen species (ROS/RNS) and to stimulate the activation of signal cascade inside the cells (Barreca, 2021). The so-called antioxidants interact directly or indirectly with the radicals and inhibit or quench free radical reactions mainly based on their reducing capacity or hydrogen atom-donating capacity. DHAP, a potentially reducing substance, and the reductive G3P are both potential antioxidants to neutralize ROS/RNS. In yeast, FBA1 was discovered to interact with the RNA polymerase III complex via coimmunoprecipitation and tRNA transcriptional regulation experiments (Ciesla et al., 2014). The cell surface-localized FBAs of Mycobacterium tuberculosis (Mtb-FBA) and genus Paracoccidioides (Pr-FBA) were demonstrated to increase virulence by binding to human plasminogen (Chaves et al., 2015; de Paz et al., 2007; Puckett et al., 2014). Furthermore, these enzymes were found to be able to activate host plasminogen to form plasmin, potentially increasing the fibrinolytic capacity of the pathogens (Chaves et al., 2015). The cell surface-localized FBA from Streptococcus suis SS9 (Ss-FBA) has been found to be immunogenic (Wu et al., 2008) and act as a host receptor for Streptococcus pneumoniae (Blau et al., 2007). FBA derived from Mycoplasma bovis (Huang et al., 2019) and Neisseria meningitides (Tunio et al., 2010a) act as antigens and interact with host cells during the process of adhesion and are thus regarded as potential therapeutic targets. In Neisseria spp., the surface-exposed FBA is highly conserved, and its surface localization and anchoring appear to be independent of its aldolase activity (Shams et al., 2016; Tunio et al., 2010a, 2010b). In Streptococcus oralis, intracellular FBA is reported to be released during exposure to alkalinity stress (de Paz et al., 2007; Wilkins et al., 2003), suggesting that FBA may play a role in physiological adaptation to environmental stress (de Paz et al., 2007; Shams et al., 2014). For higher plants, environmental alkalinization that is caused by increasing population and degradation of natural environments has become one of the most severe limitations of the development of global agriculture (Shamsabad et al., 2022). It will be of great practical importance to study the plant resistance mechanisms in alkaline stress conditions for the development of strategies for tolerance to alkalinity stresses.
In the first reported example of a native class II aldolase exhibiting reduced stereoselectivity, the thermophile Thermus caldophilus GK24-derived FBA (Tca-FBA), whose monomeric structure exhibits a typical (α/β)8 barrel, was found to produce both FBP and D-tagatose 1,6-bisphosphate (TBP; a FBP stereoisomer produced from C3 substrates such as glyceraldehyde-3-phosphate and dihydroxyacetone phosphate). Furthermore, manipulating the concentrations of G3P and DHAP was found to alter the FBP: TBP ratio (Lee et al., 2006). The Tca-FBA active site was found to be responsible for the relaxed discrimination between FBP and TBP, as it allows G3P more conformational freedom. Specifically, Asp80 was found to be primarily responsible for the chiral discrimination between FBP and TBP in Tca-FBA (Lee et al., 2006), while Asp109 in Eco. FBA appeared to significantly influence the reaction mechanism (Zgiby et al., 2000).
In Giardia lamblia, only a class II aldolase (Gl-FBA) has been identified (Galkin et al., 2007), which shares a greater sequence homology and crystal structure similarity with Ec-TBA (38%) than with Ec-FBA (23%), both derived from E. coli. Utilizing FBP as a substrate (Galkin et al., 2007), Gl-FBA employs an aspartic acid (Asp83) for its catalytic apparatus, and the activity of this enzyme was found to be halted by replacing Asp83 with an alanine residue. The active site of Gl-FBA is made up of three subsites: the DHAP binding site, the G3P binding site, and the Zn2+ binding site. Unlike Tca-FBA, Gl-FBA does not cleave TBP but recognizes TBP as a competitive inhibitor (Galkin et al., 2007, 2009). Gl-FBA is produced in the cytoplasm, and RNA silencing results in cell death (Galkin et al., 2007). Taken together, these results suggest that FBA may be a potentially useful target for the development of therapeutic small-molecule inhibitors (Shams et al., 2014).
. Function of FBA I in animals
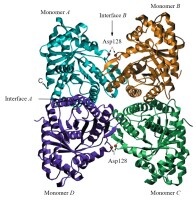
FBA derived from animals belongs to class I. In mammals, three tissue-specific FBAs have been isolated, each of which has distinct physical features: musclealdolase A (FBA Ia), liver and kidney aldolase B (FBA Ib), and brain aldolase C (FBA Ic) (Gefflaut et al., 1995). Compared to FBA Ib, FBA Ia, and FBA Ic exhibit higher activity toward PBP (Penhoet & Rutter, 1971). The amino acid sequences of these isozymes were found to be highly conserved at the subunit interface and the active site. The FBA derived from rabbit muscle was found to be a homotetramer containing two interfaces: interface A and interface B (Figure 3). Interface A is comprised of helix-packing interactions and is relatively hydrophobic, while interface B inhibited loop-loop interactions and is hydrophilic (Sherawat et al., 2008).
Figure 3
Rabbit muscle fructose-1,6-bisphophate aldolase tetramer (Sherawat et al., 2008). Each monomer is colored separately, with the A and B interfaces indicated by arrows as described previously. The sites of the D128V substitutions are indicated by red spheres on interface B, and the C- and N-termini are labeled for monomer A.
Sufficient oxygen is one of the most critical environmental conditions for animals because oxygen is crucial for oxidative phosphorylation, and insufficient oxygen results in interruption of mitochondrial ATP production (Storey & Storey, 2004). Glycolytic enzymes, such as phosphofructokinase (PFK), pyruvate kinase (PK), and lactate dehydrogenase (LDH), have been found to be important during anoxic conditions (Brooks & Storey, 1989; Willmore et al., 2001; Xiong & Storey, 2012). As a glycolytic enzyme, FBA catalyzes the conversion of FBP to G3P and DHAP, facilitating the production of ATP and lactate under anaerobic conditions. In mammals, the expression of aldolase in liver tissue typically occurs only during early development. However, under certain conditions, different aldolase heterotetramers may be upregulated, such as in turtles exposed to anoxia (Dawson et al., 2013; Lebherz & Rutter, 1969). In addition, during the long-term anaerobic condition, an overall greater affinity of liver aldolase for FBP and strict regulatory control over glycolysis (converting hexose phosphates to lactate to support the ATP requirements) made the contribution (Dawson et al., 2013). This may be the reason why the relative content of FBP in the liver doubled in T. s. elegans when exposed to the anaerobic condition for only 1 h (Kelly & Storey, 1988).
In addition to its enzymatic role, considerable evidence suggests that aldolase participates in non-metabolic processes. Aldolases are involved in binding interactions with several other proteins, although the function of these aldolases has not been fully characterized (Sherawat et al., 2008). FBA Ia and FBA Ic can act as neurofilament RNA-binding proteins, thus regulating transcriptional activity (Canete-Soler et al., 2005). FBA Ia can also bind to filamentous actin (F-actin) in vivo, playing a role in the assembly of the actin cytoskeleton (Arnold et al., 1971; Arnold & Pette, 1968; O’Reilly & Clarke, 1993). Furthermore, FBA Ia is associated with vacuolar H+-ATPases (V-ATPase) and mediates their assembly, expression, and proton pump activity (Lu et al., 2004). In the later study, it was demonstrated that the ADP-ribosylation factor (Arf) guanine nucleotide exchange factor (ARNO) directly interacted with all a-isoforms (a1–a4) of the V-ATPase. Whereafter, pull-down experiments showed that aldolase interacted with ARNO to form the aldolase -ARNO/Arf6-ATPase complex to regulate the gene expression (Merkulova et al., 2011). As a result, a novel emerging field of aldolase biology is its central role in cytoskeleton rearrangement and, cell motility and signal transduction. Aldolase has also been reported to play a role in the regulation of proteins involved in endocytosis. Sorting nexin 9 (SNX9) functions in a complex with the GTPase dynamin-2 at clathrin-coated pits to provoke the fission of vesicles to complete endocytosis. At the same time, the localization of the SNX9-dynamin-2 complex to clathrin-coated pits can be blocked by interactions with the abundant glycolytic enzyme aldolase (Lundmark & Carlsson, 2004; Rangarajan et al., 2010).
. Function of FBA I in photosynthetic plant tissues
FBAs have been identified and characterized in several plant species, including garden pea (Pisum sativum) (Anderson & Advani, 1970), maize (Zea mays) (Dennis et al., 1988; Kelley & Freeling, 1984), oats (Avena sativa) (Michelis & Gepstein, 2000), Dunaliella salina (Zhang et al., 2002), Arabidopsis (Carrera et al., 2021; Lu, 2012), soybean (Glycine max) (Russell et al., 1990), rice (Oryza sativa) (Kagaya et al., 1995; Umeda & Uchimiya, 1994; Zhang, 2014), spinach (Spinacia oleracea) (Krüger, 1983; Pelzer-Reith et al., 1993), potato (Solanum tuberosum) (Haake et al., 1998), tobacco (Nicotiana tabacum) (Yamada et al., 2000), Codonopsis lanceolate (Purev et al., 2008), Sesuvium portulacastrum (Fan et al., 2009), alfalfa (Medicago sativa) (Long et al., 2010), wheat (Triticum aestivum) (Lv, 2011; Lv et al., 2017), moso bamboo (Phyllostachys pubescens) (Lao et al., 2013), oil tree (Camellia oleifera) (Zeng et al., 2014), and tomato (Solanum lycopersicum) (Cai et al., 2016). Interestingly, several FBA I isoform members have been identified across different plant species, including 8 in Arabidopsis (AtFBA1-8) (Lu, 2012), 8 in tomato (SlFBA1-8) (Cai et al., 2016), 16 in tobacco (NtFBA1-16) (Zhao et al., 2021), 21 in wheat (TaFBA1-21) (Lv et al., 2017), and seven isoforms in rice (ALD Y and OsFBA1-6) (Zhang, 2014), indicating that contraction and expansion of the FBA gene family have occurred over the evolutionary history of these species.
Exposure to changing or stressful environmental conditions can significantly impact plant growth and development. The role of FBA in regulating the growth and development of plants has received considerable attention in recent years. Two photosynthetic tissue isoenzymes have been identified, one of which is localized to the cytosol (cFBA) and the other of which is localized to the chloroplast (ctFBA) (Anderson & Advani, 1970; Anderson & Pacold, 1972). Among the eight members in Arabidopsis, there are three members (AtFBA1–3) share high similarities with FBAs occurring at chloroplast, and five members (AtFBA4–8) share high similarities with FBAs localized in the cytoplasm (Lu, 2012). Among the eight members in tomato, there are five (SlFBA1–5) and three (SlFBA6–8) SlFBA proteins were predicted to be localized in chloroplasts and cytoplasm, respectively (Cai et al., 2016). In tobacco, phylogenetic analysis was processed, and the result revealed that these FBA genes could be categorized as subisoform I (NtFBA1–10 located in the chloroplast) and subisoform II (NtFBA11–16 located in the cytoplasm) (Zhao et al., 2021). Predicting subcellular localization fide OsFBA5 and OsFBA6 are likely to be located in the chloroplast, and the other members are likely to locate in the cytoplasm (Zhang, 2014). The activity of purified ctFBA was found to be inhibited by high concentrations of ribulose-1,5-bisphosphate (RuBP) and rescued by DHAP. That the two compartment-specific isoenzymes had no cross-reactivity was later confirmed in spinach leaves through anion-exchange chromatography on DEAE-cellulose (Krüger, 1983). Specifically, chloroplast-derived aldolase was found to be responsible for 85% of the total activity, while cytosol-derived aldolase was found to be responsible for only 15% of spinach leaves. Furthermore, cross-reaction with anti-(carrot ctFBA)-IgG was detected between cFBA and ctFBA (Moorhead & Plaxton, 1990), providing an effective method for the separation of these two types of FBAs for functional characterization. Utilizing this technique, 8 FBAs were identified in Arabidopsis, which were found to be responsive to glucose, fructose, sucrose, phytohormones, and environmental stress (Lu, 2012).
More evidence that FBA contributes to regulating growth and development is found in Arabidopsis. Although Atfba2 mutants exhibited a slow growth rate, their overall development remained normal. However, Atfba1 mutants exhibited no such phenotype, although the FBAs shared high homology. A combination of Atfba1 and Atfba2 mutants exhibited inhibition of photo-autotrophy, which proved to be lethal. AtFBA3 is predominantly expressed in heterotrophic tissues and affects photo-assimilate export, leading to growth inhibition (Carrera et al., 2021). Furthermore, fba8 mutant Arabidopsis, which encodes a cytosolic localized protein, exhibited significantly reduced FBA activity in roots as well as a reduced growth rate (Garagounis et al., 2017). In Arabidopsis, AtFBA6 encodes a cytoplasmic localized protein. The expression of AtFBA6 is positively regulated by sugar and universally expressed, especially in the shoot apical meristem (SAM), root apical meristem (RAM), and vascular bundles (Fan, 2020). Overexpression of AtFBA6 resulted in smaller plants with smaller, thinner leaves and advanced floral bud differentiation (Lu, 2012). In addition, slower leaf and root development was observed in Atfba6 mutants (Fan, 2020). Similar phenotypes were observed in both mutant and overexpression plants, likely due to functional redundancy among FBA family members. AtFBA6 has been found to interact with WUSCHEL (WUS), WUSCHEL-RELATED HOMEOBOX 4 (WOX4), and WOX5, and positively regulate the expression of WUS, WOX4, and WOX5. WUS, whose expression is positively regulated by O2- (Schuster et al., 2014), regulates and stabilizes SAM stem cells and is involved in floral meristem (FM) morphogenesis (Ikeda et al., 2009). These studies provided a very bright light that FBA participated in plant morphogenesis.
Under salt stress, the unicellular green alga Dunaliella salina can synthesize glycerol as an osmolyte. Dihydroxyacetone phosphate, produced from a reaction catalyzed by FBA, is a precursor of glycerol synthesis under salt stress, suggesting that FBA may be stress-responsive and confer resistance to stressors such as salt and extreme temperatures. Furthermore, during glycolysis, aldolase activity can improve the production of pyruvate, which is converted to α-ketoglutaric acid in the tricarboxylic acid (TCA) cycle. α-ketoglutaric acid is a precursor of the synthesis of proline, another osmolyte that protects plant cells during stressful conditions (Zhang et al., 2003). In 1997, a fructose 1,6-bisphosphate aldolase was identified in a rice mutant, conferring increased production of lysine and protein. However, the differences in enzymatic activity were attributed to physiological adjustments rather than DNA modifications of the aldolase gene(s) (Schaeffer et al., 1997).
Studies on C. oleifera suggest that FBA plays a vital role in regulating secondary metabolism (Zeng et al., 2014). To date, different isoforms of FBA have been found to exhibit tissue-specific expression levels (Carrera et al., 2021), and evidence is mounting that ctFBA/cFBA may exert significant metabolic control in vivo. It is well known that the Calvin cycle provides intermediates for glycolysis or building blocks for cellular components, thus playing an indispensable role in plant growth and maintenance (Uematsu et al., 2012). In the past decades, research into the rate-limiting steps of the Calvin cycle has focused on genetic manipulation for the enhancement of photosynthetic capacity and plant productivity. In higher plants, aldolases are involved in photosynthetic carbon flux and the regulation of plant growth and stress assistance. Heterologous expression of Arabidopsis plastid FBA in tobacco resulted in increased plastic FBA activity, growth rate, accumulation of aerial tissue biomass, CO2 fixation rate, content of RuBP, photosynthetic rate, and starch accumulation (Uematsu et al., 2012). However, it was unclear how the increased aldolase activity could promote RuBP regeneration or whether the mechanism involved the regulation of SBPase or not. In 2017, research on tomatoes (Solanum lycopersicum) indicated that plastid-localized aldolase activity regulated the activities of enzymes involved in the Calvin cycle (Cai, 2017). Further studies in tomatoes indicated that not only ctFBA but also cFBA play pivotal roles in plant growth and tolerance to low-temperature stress (Cai et al., 2016, 2018). In potatoes, gene silencing techniques were utilized to reduce FBA activity, resulting in a marked inhibition of photosynthetic CO2 fixation and growth (Haake et al., 1998). Later on, the interaction of FBA6 and calmodulin-like proteins (CMLs) was verified in alfalfa (Medicago sativa) (Yu et al., 2022). Ca2+ channel was activated in plants when exposed to low-temperature conditions, leading to a transient rise of free Ca2+ in the cytosol that can be perceived by CML, a type of Ca2+ sensor (Ding et al., 2019). CML10 in Medicago sativa (MsCML10) decodes the cold-induced Ca2+ signal and regulates cold tolerance through activating MsFBA6, leading to increased accumulation of sugars for osmoregulation. Not only in low-temperature stress conditions but during water deficit, the protein abundance level of FBA and the enzymatic activity increased when water loss to 50% relative water content (Kamies et al., 2017). These results suggest that differentially localized FBAs may exhibit different characteristics. In varies plants, FBA takes participant in multiple abiotic stresses tolerance through varies pathways.
. Function of FBA I in non-photosynthetic plant tissues
FBAs derived from vascular plants belong to class I and are homotetramers (Lal et al., 2005). While it has not yet been established whether tissue-specific aldolase isozymes occur in vascular plants, different types of FBAs have been identified in photosynthetic and non-photosynthetic plant tissues and are encoded by different nuclear genes which are likely derived from a common ancestral gene, including the cytosolic FBA (cFBA) and chloroplast/plastid FBA (ctFBA) (Cai et al., 2016; Plaxton, 1996; Tsutsumi et al., 1994; Yamada et al., 2000). The cytosolic aldolase has been found to contain larger subunits than the chloroplast aldolase (Krüger, 1983), and they are compartmentalized as isozymes and are involved in different metabolic reactions. In many algae, only one type of isozyme has been detected (Schnarrenberger et al., 1994; Zhang et al., 2003). Different thermal stabilities, net charges, amino acid compositions, immunological properties, and subunit sizes have been observed between cFBA and ctFBA. Some studies suggest that ctFBA contributes much more to non-photosynthetic tissues than to photosynthetic tissues, often contributing more than 50% of the total FBA activity (Botha & O’Kennedy, 1989; Hodgson & Plaxton, 1998; Krüger & Schnarrenberger, 1985; Moorhead & Plaxton, 1990; Nishimura & Beevers, 1981; Schwab et al., 2001).
The function of FBA in non-photosynthetic tissues has been studied in germinating common beans (Phaseolus vulgaris), Castor (Ricinus communis) seeds, carrot (Daucus carota) roots, and germinating mung beans (Vigna radiata) (Botha & O’Kennedy, 1989; Hodgson & Plaxton, 1998; Lal et al., 2005; Moorhead & Plaxton, 1990). In germinating P. vulgaris seeds, ctFBA, but not cFBA, was found to not bind to phosphocellulose, resulting in easy separation of ctFBA from cFBA (Botha & O’Kennedy, 1989). A similar phenomenon was observed in spinach leaves (Krüger, 1983). Purified FBA lost only 25% of its activity when incubated for 5 minutes at 65 °, suggesting that the enzyme is relatively heat-stable (Lal et al., 2005). In germinating mung beans, as well as other plants, cFBA activity appears to be heat stable (Botha & O’Kennedy, 1989; Hodgson & Plaxton, 1998; Moorhead & Plaxton, 1990; Schwab et al., 2001). By contrast, ctFBA activity can be completely inactivated by heat treatment, suggesting that ctFBA is heat labile (Lebherz & Rutter, 1969; Schwab et al., 2001). Different substrate specificities have been observed in FBAs derived from different plants and plant tissues. In germinating mung beans, cytosolic FBA plays a bifunctional role in catalyzing the aldol cleavage of FBP and SBP, similarly to cytosolic FBAs of other plants (Botha & O’Kennedy, 1989; Hodgson & Plaxton, 1998; Lal et al., 2005; Lebherz & Rutter, 1969; Moorhead & Plaxton, 1990; Schnarrenberger & Krüger, 1986; Schwab et al., 2001). However, neither F1P nor F6P could serve as the substrate for either mung bean cytosolic FBA or carrot cytosolic FBA (Lal et al., 2005; Moorhead & Plaxton, 1990). In other plants and plant tissues, including pea (Pisum sativum), wheat (Triticum aestivum), corn leaf (Zea mays), and castor (Ricinus communis), cytosolic FBA exhibited activity at high concentrations of F1P (Hodgson & Plaxton, 1998; Schnarrenberger & Krüger, 1986).
Research on FBA in vivo suggests that the enzyme exerts metabolic control on photosynthetic CO2 fixation and growth (Cai et al., 2018, 2022; Haake et al., 1998). Like the class II FBAs isolated from pathogenic microorganisms, in carrot FBA I can physically interact with other glycolytic or gluconeogenic enzymes, including cytosolic ATP- and PPi-dependent phosphofructokinases, in vivo (Moorhead & Plaxton, 1992). In R. communis endosperm and mammalian liver tissue, FBA I can interact with cytosolic FBPase during gluconeogenesis (Moorhead et al., 1994; Pontremoli et al., 1979). Furthermore, in Arabidopsis, FBA has been reported to be physically associated with the cytosolic side of the outer mitochondrial membrane (Giege et al., 2003). The in vitro activity of FBA purified from both animals and plants can be modulated by physiologically relevant concentrations of various metabolite effectors (Akkerman, 1985; Botha & O’Kennedy, 1989; Hodgson & Plaxton, 1998; MacDonald & Storey, 2002; Moorhead & Plaxton, 1990). It is well known that FBA is one of the six enzymes whose activity is not regulated by effectors or post-translational modification. Through expressional regulation or protein degradation during the Calvin cycle (Graciet et al., 2004), neither dithiothreitol, Pi, P-enolpyruvate, 3-P-gluconate, citrate, glucose, glucose-1-P, glucose-6-P, fructose, ribose, arabinose, adenosine, nor sucrose (5 mM each), or fructose-2,6-P2 (50 µM), were found to alter enzymatic activity. However, ribose-5-P, AMP, ADP, and particularly ATP, were found to effectively inhibit enzymatic activity (Lal et al., 2005).
. Differences between FBA and FBA/P
Because many archaea lack a functional fructose 1,6-bisphosphate aldolase, it is not fully understood how gluconeogenesis functions in these organisms. Recently, a novel bifunctional FBP aldolase-phosphatase (FBA/P) was discovered, and could be the ancestral gluconeogenic enzyme (Berg et al., 2010). FBA/P is a bifunctional, thermostable enzyme encoded in the genomes of virtually all archaea, as well as deeply branching bacteria. Although this enzyme possesses both aldolase and phosphatase activity, it has been found to be involved in gluconeogenesis, rather than in glycolysis. FBA/P exhibits higher activity in the condensation reaction than in the FBP cleavage reaction (Say & Fuchs, 2010). Bifunctional enzymes generally consist of either two distinct catalytic domains or a single domain exhibiting promiscuous substrate specificity (Moore, 2004). FBA/P can consecutively and dramatically change its conformation to reorganize its active center and perform two drastically different catalytic steps in a highly controlled and ordered sequence. The archaeal fructose-1,6-bisphosphate aldolase/phosphatase (FBA/P) consists of a single catalytic domain but catalyzes two chemically distinct gluconeogenic reactions, making it fundamentally different from ordinary enzymes whose active sites are responsible for a specific reaction. Although FBA/P appears to be physiologically unrelated to any known aldolase (St-Jean et al., 2009), it exhibits an FBA I-like mechanism involving a lysine Schiff-base with DHAP (Grazi et al., 1962; Rose & Rieder, 1958). However, despite the similar mechanism, the FBA/P Km for the aldol condensation and cleavage differ by a factor of 1,000 (Say & Fuchs, 2010).
. Conclusion and expectations
FBAs are widely distributed across many clades and are essential for anabolism, catabolism, and process regulation. In recent years, a considerable amount of research has been conducted on the function of FBA in bacteria and fungi in the hope of developing a therapeutic target against pathogen infection. However, less research has been conducted on the function of FBA in higher plants, including under normal and stress conditions. To address this knowledge gap, we suggest the following questions as potential topics of inquiry: (1) Does FBA directly bind to other proteins or cell factors? (2) Does FBA interact with any proteins other than WUS, WOX4, and WOX5? (3) Does FBA interact with any downstream response factors other than WUS, WOX4, and WOX5? (4) What is the upstream regulator of FBA? (5) What roles do the upstream regulators and downstream response factors of FBA play in the abiotic stress response? (6) Does FBA participate in the regulation of flower bud differentiation in higher plants? Answering these questions and others will further our understanding of this interesting enzyme.


