. Introduction
Gene banks are international collections that collect, conserve, and make available accessions of crops, crop wild relatives, tree species, and endangered plants using procedures and conditions that uphold the highest standards. They play an important role in advancing plant breeding programs (Ochatt et al., 2021) and protecting endangered species (Pence, 2011). Traditional tools of in situ and ex situ conservation are supplemented by cryopreservation techniques applied for the long-term storage of plant germplasms. Cryopreservation involves the cooling and storing of vegetal structures, such as seeds and pollen, as well as plant cells, tissues, or organs, in liquid nitrogen (LN; −196 °C) or LN vapor (from −130 °C up to −160 °C). According to mathematical models, this method ensures the maintenance of plant material viability for a long period of time (over 500 years) (Walters et al., 2005). LN can be used as a backup for the collection of clonally propagated plant species, and plant gene banks are increasingly using cryopreservation technologies to secure vegetatively propagated collections as secondary locations (M.-R. Wang et al., 2021). LN is also recommended for the storage of recalcitrant (non-orthodox) seeds, which are unsuitable for in vitro maintenance and long storage in traditional seed banks (Reed, 2008). Moreover, cryopreservation is applied to store seeds of rare and endangered plant species, regardless of their storage capacity (Cruz-Cruz et al., 2013; Engelmann, 2011), with seeds typically stored at −20 °C.
To date, plant gene banks have poorly implemented LN for long-term storage despite the development of cryopreservation techniques for several hundreds of plant species (Benelli, 2021). This may be due to the high cost of purchasing in vitro equipment (Mikuła et al., 2013), training personnel in cryopreservation procedures adapted to specific species, and some critical factors in the cryotechnology affecting its effectiveness, including the type of plant material, conditions of preculture, applied techniques, conditions of cooling, rewarming, and regrowth (Bettoni et al., 2021). However, cryopreservation has several advantages over the maintenance of living collections, including relatively low cost of long-term storage (Keller et al., 2013), limited labor consumption, and reduced occurrence of contamination associated with traditional in vitro maintenance of plants, which minimizes the risk of loss of the stored germplasm ensures and high genetic stability of the stored accessions due to the inhibition of life processes at ultra-low temperatures (Cruz-Cruz et al., 2013; Reed, 2008).
The commonly used techniques for cryopreservation are classical freezing with a slow cooling regime (Kartha & Engelmann, 1994), vitrification (Sakai & Engelmann, 2007), encapsulation-dehydration (Fabre & Dereuddre, 1990), dormant bud preservation (Forsline et al., 1998), and others that are typically modifications of the methods listed above (Benelli, 2021). Several procedures based on mixed cryotechniques have been developed, including the use of various types of plant propagules (M.-R. Wang et al., 2021). The type of plant propagule depends on the plant species and further use of plant material, including seeds, pollen, dormant buds, shoot tips, embryonic axes, plumules, zygotic or somatic embryos, gametophytes, callus, or cell suspension cultures (Chmielarz et al., 2011; Pence, 2000; Reed, 2008).
To date, the global use of LN in gene banks has been limited. Benelli (2021) estimated that in plant collections around the world, there are currently about 10,000 accessions stored in LN, whose explants were derived from in vitro plants. Furthermore, these collections (80%) are comprised of only a few crop species (potato, banana, cassava, garlic, and mulberry). In addition, a few thousand accessions of apple and pear have been cryopreserved in the form of dormant buds (Jenderek & Reed, 2017). Dormant bud cryopreservation was recommended for some tree fruit crops by Tanner et al. (2021) as an alternative to the labor-intensive procedure of shoot tip cryopreservation.
The objective of this review is to summarize the current applications of LN in plant genetic resource conservation in Poland.
. Ex Situ Plant Protection Programs Implemented in Poland
National Program for the Protection of Crop Plants Genetic Resources (since 1996) (https://bankgenow.edu.pl/). This program aims to protect the genetic material of crop plants, non-cultivated plants, and their wild relatives (CWR) for breeding, scientific research, and agriculture. The National Center for Plant Genetic Resources (NCPGR): Polish GenBank is located at the Plant Breeding and Acclimatization Institute, National Research Institute (PBAI-NRI). The NCPGR is the coordinator of the national plant genetic resources (including the cryopreserved genetic resources of apple, garlic, and potato described in this review). EGISET, a database system for documentation of NCPGR collections, was implemented in 2010. It enables the effective care of over 86,000 resources collected mainly as seed samples stored at 0 °C and −18 °C at PBAI-NRI. Among the >1,800 potato accessions maintained in vitro, 165 are cryopreserved.
The European Native Seed Conservation Network (ENSCONET) (2004–2009) (https://cordis.europa.eu/project/id/506109/reporting). This program was carried out in collaboration with 30 institutions from 18 countries. Poland was represented by the Polish Academy of Sciences Botanical Garden – Center for Biological Diversity Conservation in Powsin, Warsaw (PAS BG-CBDC) as a leader of the activity group “seed curation.” Seeds of 110 species from 350 populations were collected. The project resulted in the establishment of a network of ex situ collections of wild species and the development of seed storage and germination methodologies. At the end of the project, in October 2009, a virtual seed bank (ENSCOBASE; http://enscobase.maich.gr/) comprised of entries from 29 seed banks across Europe, representing 39,292 accessions from 8,973 taxa native to Europe and 11 biogeographical regions in Europe, was generated (Rivière & Müller, 2018). By 2022, ENSCOBASE will include 78,607 accessions.
Vegetative Allium L., Europe’s Core Collection, Safe and Sound (EURALLIVEG) (2007–2011) (http://euralliveg.ipk-gatersleben.de/home.php). This project was funded by the European Commission Directorate-General for Agriculture and Rural Development and coordinated by the Leibniz Institute of Plant Genetics and Crop Plant Research Gatersleben (IPK). The main focus of the project was the development of a European integrated Allium L. core collection, including the national collections of the Czech Republic, France, Germany, Italy, Poland, and Nordic countries. The Cryobanks Network was organized by three project partners: the Czech Republic, Germany, and Poland. Accessions were maintained in at least two locations, that is, in the host gene bank and one of the partner’s gene banks. During the project period, 34 non-bolting garlic accessions were described, of which 20 ones fulfilled EURALLIEVEG safety standards for long-term storage (Olas-Sochacka & Kotlińska, 2015). A part of the garlic collection with the highest priority was examined for infestation with five viruses (Keller et al., 2012). As a result of this project, 147 accessions were deposited in the Polish cryobank. This study aimed to develop comprehensive strategies for the long-term protection of the gene pool of species that are endangered by extinction and the plants from the natural habitats in Poland and Natura 2000 areas. As a result, the seeds of 58 species from 129 sites in Poland were stored in LN.
FlorNaturLBG (2009–2012) (http://flornaturlbg.pl/). This was a program for the ex situ conservation of threatened and protected native plant species in the western part of Poland, implemented in the Kostrzyca Forest Gene Bank (Kostrzyca FGB). It aimed to develop a strategy for the comprehensive long-term protection of the gene pool of species that are endangered by extinction, namely plants from the natural habitats in Poland and the Natura 2000 areas. The seeds of 58 species from 129 sites in Poland were stored in LN.
FlorNaturOB (2010–2013) (https://ogrod-powsin.pl/nauka/projekty/projekt-flornaturob/). This program was characterized by the ex situ conservation of threatened and protected native plants in the central and eastern part of Poland. The project was developed at the PAS BG-CBDC. As a result, seeds of 61 species of vascular flora that are threatened by extinction were collected at 161 natural sites in Poland and preserved in LN.
FlorNatur ROBiA (2013–2015) (https://ogrod-powsin.pl/nauka/projekty/projekt-nfos-flornatur-robia/). As a result of the project “Population assessment and ex situ conservation of selected wild-growing rare and endangered plant species in Poland – FlorNatur ROBiA,” a network of regional seed banks was created and the seeds of 33 protected, rare, or endangered plant species were collected from 75 sites across Poland. The project was implemented by the Council of Botanical Gardens in Poland in five botanical gardens: Maria Curie-Skłodowska University Botanical Garden in Lublin, Silesian Botanical Garden in Mikołów, PAS BG-CBDC, Adam Mickiewicz University Botanical Garden in Poznań, and Kostrzyca FGB. As a result, a national network of native flora seed banks was established.
FlorIntegral (2018–2021) (https://florintegral.pl/). The aim of the project was the active conservation of communities and species of the Polish flora through the implementation of a large-scale plan of in situ and ex situ integrated active conservation of selected species and their habitats. The project consolidated and, in a complementary way, combined a spectrum of good practices applied in active nature conservation based on knowledge gained from previous research on the effective conservation of genetic diversity of selected species of high conservation status in Poland.
. Cryobanks in Poland
Plant cryopreservation is a widely applied technique for storing vegetal structures, such as plant cells, tissues, and organs (Benelli, 2021). Cryopreservation protocols have also been established for wild flora seed conservation. However, the large-scale, routine application of cryopreservation remains limited in comparison to conventional low-temperature seed storage (typically −20 °C). There are four cryobanks in Poland, of which two have specialized mainly in long-term seed storage (Section 3.1–Section 3.4).
The Polish Academy of Sciences Botanical Garden – Center for Biological Diversity Conservation in Powsin (PAS BG-CBDC)
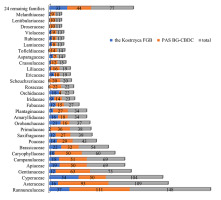
In 1992, PAS BG-CBDC established the first wild flora seed bank in Europe for plant diversity conservation based on the cryogenic technique. The seed bank focuses mainly on the collection and storage of seeds of the most threatened and endangered plant species in Poland, collected from natural sites, to create a reserve of plant genetic resources in case of their extinction. Long-term seed storage methods are based on standardized protocols developed by botanical gardens worldwide in cooperation with the Royal Botanic Gardens, Kew. However, nearly all protocol steps have been re-optimized for many species with the knowledge of dormancy breaking and freezing tolerance of the seeds (Niemczyk & Puchalski, 2015; Puchalski et al., 2014). A germination test was performed for each set of seeds collected in the field and delivered to the seed bank before storage in LN and after 15–20 years of cryopreservation for controlled seed samples. Non-germinating seeds were also tested using a tetrazolium test to separate dead from viable but dormant seeds. As a result of several European and national projects conducted in the last 15 years, 241 endangered, rare, and legally protected species of Polish flora were collected from 885 localities and preserved as cryogenic seed bank accessions (Table S1). Substantial and unique taxonomic diversity exists among the seed bank holdings, representing 48 herbaceous plant families. Ranunculaceae, Asteraceae, and Gentianaceae were the most abundant families, covering 111, 93, and 63 accessions, respectively. A group of 27 families was represented by 10 or less accessions per family, including 14 with single accessions (Figure 1). Cryo-collection possesses significant national conservation value, containing 125 species under strict protection and 16 under partial protection, according to the Polish regulation of the Minister of the Environment released on October 9, 2014, on the conservation of plant species (Regulation of the Minister of Environment, 2014).
Figure 1
Number of accessions of individual herbaceous plant families whose seeds are cryopreserved in Polish cryobanks: Kostrzyca Forest Gene Bank (Kostrzyca FGB) and Polish Academy of Sciences Botanical Garden – Center for Biological Diversity Conservation in Powsin, Warsaw (PAS BG-CBDC). The class of “remaining families” refers to the groups of families with less than 10 accessions each.
In the framework of the FlorIntegral project, the cryopreserved plant material of the threatened species Adenophora liliifolia (L.) Besser was used for its successful restitution in the Kampinoski National Park as an example of the integration of in situ and ex situ conservation efforts. Moreover, as an effect of the FlorIntegral project, a DNA Bank was established to provide a backup for traditional ex situ storage. The plant material for DNA banking was collected simultaneously with seeds from a particular population, and the extracted genomic DNA was stored at a deep-freezing temperature of −80 °C. This repository consists of accessions available for the study of population diversity and the relationships among plant taxonomic groups.
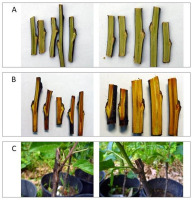
LN was also used in PAS BG-CBDC to preserve the germplasm of apple trees. The winter dormant buds of historical apple tree varieties (Malus domestica Borkh.) have been cryopreserved since 2009, starting with a project financed by the National Center for Research and Development for the establishment of such a collection. Currently, 289 apple varieties have been stored in vapor LN temperature (−150 °C) with at least 50 buds each (Table S2). Each year, the collection is expanded by another 15–20 varieties. The freezing method is based on a slow cooling regime, with some modifications (Woliński et al., 2011). The average recovery level of dormant buds of the varieties stored for 12 seasons as thawed buds grafted onto rootstocks was as high as 68% (Figure 2).
Figure 2
Dormant buds of historical apple tree varieties: ‘Spasówka’ variety (left) and ‘Ingrid Marie’ variety (right). (A) Non-LN-stored control with bright green stems and buds. (B) Storage in LN visibly changes the stem and buds color to dark yellow, which is not associated with the regeneration capacity of buds. (C) Regenerated thawed buds grafted on rootstocks: ‘Lady Sudeley’ variety (left) and ‘Jaster’ variety (right).
Moreover, distinct and adapted cryogenic techniques have been applied to a wide range of tissues, such as embryogenic tissue cultured in a liquid medium and independent-living autotrophic fern gametophytes, which are capable of sexual reproduction and vegetative propagation, especially under in vitro culture conditions (Rybczyński et al., 2018). Efficient in vitro regeneration systems developed at the Department of Plant Conservation Biology of the PAS BG-CBDC (Rybczyński et al., 2015, 2018) have provided sufficient tissue for experimental purposes, thus enriching our understanding and enabling the development of highly efficient cryopreservation protocols (Makowski et al., 2015, 2016; Mikuła et al., 2011, 2015; Tomiczak et al., 2018). This has allowed for the embryogenic tissues of four species of the genus Gentiana to be introduced into LN and stored successfully for 11 years (Table 1). The cryopreservation of gametophytes has proven to be an excellent method for supporting the long-term conservation of ferns. Multi-stage cryogenic protocols have been established for the cryopreservation of gametophytes propagated in in vitro cultures. It is worth noting that strategies for active protection of ferns, both in the world and Poland, are poorly developed. Few publications have described procedures for the routine collection and storage of fern spores in germplasm banks (Ballesteros & Pence, 2018). In fact, cryopreservation methods, have been developed for gametophytes propagated in vitro for only 25 fern species belonging to 15 genera (Table S3). Among them, 15 species are successfully protected at the cryobank of PAS BG-CBDC. Their tissues remain at cryogenic temperature for 10–13 years with a high regeneration capacity (Table 1). Nine species have been classified as tree ferns. Thus, the world’s largest cryo-collection of nine tree fern species has been established in Poland. However, the ex situ conservation of Polish pteridophyte flora is limited to a few local species in the living collections of botanical gardens, including PAS BP-CBDC. None of the ex situ plant conservation programs in Poland include spore plants.
Table 1
Cryopreservation procedures of plant material (excluding seeds) developed in Poland for plant species accessions stored for long term in liquid nitrogen or its vapors.
| Plant species | Type of plant material | Technique1 | No. of genotypes/accessions | Average survival/range (%) | Stored in2 | References |
|---|---|---|---|---|---|---|
| Seed plants | ||||||
| Allium sativum L. | Shoot tips | V | 228 | >30.0 | InHort | Chojnowski p.c., 2022; Olas-Sochacka (2017) |
| Ajania pacifica (Nakai) Bremer & Humphries | Shoot tips | E-D | 1 | 77.8 | PBS | Kulus and Abratowska (2017) |
| Chrysanthemum ×morifolium (Ramat.) Hemsl. | Shoot tips | E-D | 3 | 54.1/42.5–66.7 | PBS | Kulus et al. (2018) |
| Fraxinus excelsior L. | Dormant buds | PF | 47 | Kostrzyca FGB | Towill and Ellis (2008) | |
| Gentiana cruciata L. | PEM3 | E-D | 1 | 83 | PAS BG-CBDC | Mikuła et al. (2008) |
| G. kurroo Royle | PEM | E-D | 1 | 95 | PAS BG-CBDC | Mikuła et al. (2011) |
| G. pannonica Scop. | PEM | E-D | 1 | 70 | PAS BG-CBDC | Mikuła p.c., 2022 |
| G. tibetica King ex Hook. f. | PEM | E-D | 1 | 68 | PAS BG-CBDC | Mikuła et al. (2008) |
| Lamprocapnos spectabilis (L.) Fukuhara | Shoot tips | E-D | 1 | 36.4 | PBS | Kulus (2020) |
| Shoot tips | E-V | 3 | 65/53–73 | PBS | Kulus and Miler (2021); Kulus and Tymoszuk (2021) | |
| Malus domestica Borkh. | Dormant buds | PF | 289 | 68 | PAS BG-CBDC | Woliński et al. (2011) |
| Solanum tuberosum L. 4x | Shoot tips | V | 4 | 67.5/42–88 | PBAI-Młochów RC | Kryszczuk et al. (2006) |
| Pollen | DF | 17 | nt | Smyda-Dajmund p.c., 2022 | ||
| S. tuberosum L. hybrids 2x | Shoot tips | V | 56 | 38.5/10–70 | PBAI-Młochów RC | Smyda-Dajmund (2017) |
| Pollen | DF | 96 | nt | |||
| S. phureja Juz. et Buk. | Pollen | DF | 4 | nt | PBAI-Młochów RC | Smyda-Dajmund p.c., 2022 |
| S. kurtzianum Bitter & Wittm. | Pollen | DF | 2 | nt | PBAI-Młochów RC | Smyda-Dajmund p.c., 2022 |
| S. ruis-ceballosi Card. | Pollen | DF | 3 | nt | PBAI-Młochów RC | Smyda-Dajmund p.c., 2022 |
| S. pinnatisectum Dunal | Pollen | DF | 4 | nt | PBAI-Młochów RC | Smyda-Dajmund p.c., 2022 |
| S. michoacanum (Bitter) Rybd. | Pollen | DF | 7 | nt | PBAI-Młochów RC | Smyda-Dajmund p.c., 2022 |
| Quercus robur L. | Plumules | V | 534 | 51–76 | Kostrzyca FGB | Chmielarz et al. (2011) |
| Spore plants | ||||||
| Asplenium adulterinum Milde | Gametophytes | E-D | 1 | 100 | PAS BG-CBDC | Makowski (2013) |
| A. adiantum-nigrum L. | Gametophytes | E-D | 1 | 100 | PAS BG-CBDC | Makowski (2013) |
| A. cuneifolium Viv. | Gametophytes | E-D | 1 | 100 | PAS BG-CBDC | Makowski (2013) |
| A. scolopendrium L. | Gametophytes | E-D | 1 | 100 | PAS BG-CBDC | Mikuła et al. (2011) |
| Ceratopteris cornuta (P. Beauv.) Lepr. | Gametophytes | E-D | 1 | 95 | PAS BG-CBDC | Mikuła p.c., 2022 |
| C. thalictroides (L.) Brongn. | Gametophytes | E-D | 1 | 91 | PAS BG-CBDC | Makowski et al. (2015) |
| Cibotium glaucum (Sm.) Hook. & Arn. | Gametophytes | E-D | 1 | 82 | PAS BG-CBDC | Mikuła et al. (2011) |
| C. schiedei Schltdl. & Cham. | Gametophytes | E-D | 1 | 90 | PAS BG-CBDC | Mikuła et al. (2011) |
| Cyathea australis Domin. | Gametophytes | E-D | 1 | 83 | PAS BG-CBDC | Mikuła et al. (2011) |
| C. dealbata (G. Forst.) Sw. | Gametophytes | E-D | 1 | 68 | PAS BG-CBDC | Mikuła et al. (2011) |
| C. delgadii Sternb. | Gametophytes | E-D | 1 | 92 | PAS BG-CBDC | Mikuła et al. (2011) |
| C. smithii Hook. f. | Gametophytes | E-D | 1 | 79 | PAS BG-CBDC | Mikuła et al. (2011) |
| Dicksonia fibrosa Colenso | Gametophytes | E-D | 1 | 99 | PAS BG-CBDC | Mikuła et al. (2011) |
| D. sellowiana (Pr.) Hook. | Gametophytes | E-D | 1 | 83 | PAS BG-CBDC | Mikuła p.c., 2022 |
| Osmunda regalis L. | Gametophytes | E-D | 1 | 82 | PAS BG-CBDC | Mikuła et al. (2011) |
1 E-D – encapsulation-dehydration technique; E-V – encapsulation-vitrification technique; V – vitrification technique; PF – programmed freezing; DF – direct freeze; p.c. – personal communication; nt – not tested.
2 PAS BG-CBDC – Polish Academy of Sciences Botanical Garden – Center for Biological Diversity Conservation in Powsin, Warsaw; PBAI-Młochów RC – Plant Breeding and Acclimatization Institute – National Research Institute, National Center for Plant Genetic Resources, Młochów Research Center; PBS – Bydgoszcz University of Science and Technology; Kostrzyca FGB – Kostrzyca Forest Gene Bank; InHort – Research Institute of Horticulture – National Research Institute in Skierniewice.
The Kostrzyca Forest Gene Bank (Kostrzyca FGB)
The second Polish cryobank is Kostrzyca FGB, which has been active since 1995 and has focused on the conservation of forest fauna and flora, particularly forest tree species. Kostrzyca FGB has introduced LN storage as a routine application in the seed collection of several forest species, based on protocols developed by the research team of the Institute of Dendrology, PAS (Chmielarz, 2007, 2009a, 2009b, 2010a, 2010c, 2010d; Chmielarz et al., 2011; Michalak, Plitta-Michalak, & Chmielarz, 2015). In the forest cryobank, 1,226 accessions of forest trees and the shrubs of 33 species belonging to 20 genera are cryopreserved (Table 2). Among the cryopreserved species, seven are under strict protection according to the Polish regulation of the Minister of the Environment on October 9, 2014 (Regulation of the Minister of Environment, 2014).
Plumules (apical meristem of the embryonic axis) of oaks [Quercus robur L. and Q. petraea (Matt.) Liebl.] from 115 accessions were preserved in LN according to the protocol described by Chmielarz et al. (2011). Dormant buds of common ash (Fraxinus excelsior L.) from 47 accessions were frozen following the protocol by Towill and Ellis (2008), along with 98 seed samples of this species frozen in LN according to the protocol modified by Chmielarz (2009a). Tree species with the most numerous cryopreserved accessions were found to be among the genera Abies Mill., Alnus Mill., Betula L., Ulmus L., Quercus L., and Fraxinus Tourn. ex L. Seeds of the genera Rhododendron L., Salix L., Sambucus L., Sorbus L., and Taxus L. were frozen in cryogenics after seed drying treatment optimized for each species, developed at Kostrzyca FGB (M. Pałucka, personal communication, 2022) (Table 2).
Seeds of high quality and viability should be used for cryogenic storage. Therefore, at Kostrzyca FGB, at the local Seed Assessment Station, a full laboratory evaluation of 1,000 seeds (germination or TTC tests), including evaluation of purity and weight, was performed before the creation of cryogenic resources.
Table 2
Seed accessions of forest trees and shrubs cryopreserved at the Kostrzyca Forest Gene Bank.
| Plant genus | Type of plant material | Technique1 | Number of accessions | References |
|---|---|---|---|---|
| Abies Mill. | Seeds | DF | 295 | Chmielarz (2007)2 |
| Alnus Mill. | Seeds | DF | 265 | Chmielarz (2010a)2 |
| Betula L. | Seeds | DF | 156 | Chmielarz (2007)2 |
| Carpinus L. | Seeds | DF | 1 | Chmielarz (2010d)2 |
| Daphne Tourn. ex L. | Seeds | DF | 1 | Pałucka p.c., 20223 |
| Dictamnus Zinn. | Seeds | DF | 1 | Pałucka p.c., 20223 |
| Frangula Mill. | Seeds | DF | 1 | Pałucka p.c., 20223 |
| Fraxinus Tourn. ex L. | Seeds | DF | 98 | Chmielarz (2009a)2 |
| Dormant buds | PF | 47 | Towill and Ellis (2008) | |
| Hippophae L. | Seeds | DF | 1 | Pałucka p.c., 20223 |
| Malus Mill. | Seeds | DF | 1 | Michalak, Plitta-Michalak and Chmielarz (2015)2 |
| Pinus L. | Seeds | DF | 2 | Chmielarz (2007) |
| Prunus L. | Seeds | DF | 29 | Chmielarz (2009b)2 |
| Quercus L. | Plumules | V | 115 | Chmielarz et al. (2011) |
| Rhododendron L. | Seeds | DF | 3 | Pałucka p.c., 20223 |
| Salix L. | Seeds | DF | 3 | Pałucka p.c., 20223 |
| Sambucus L. | Seeds | DF | 1 | Pałucka p.c., 20223 |
| Sorbus L. | Seeds | DF | 8 | Pałucka p.c., 20223 |
| Taxus L. | Seeds | DF | 2 | Pałucka p.c., 20223 |
| Tilia L. | Seeds | DF | 34 | Chmielarz (2007) |
| Ulmus L. | Seeds | DF | 162 | Chmielarz (2007, 2010c) |
| 20 genera | 1,226 |
The second group of cryopreserved seeds in Kostrzyca FGB is comprised of 194 herbaceous species, belonging to 41 botanical families, mainly rare and endangered plants from the habitats of Polish forests and meadows. There are 372 accessions in this group, with the families Cyperaceae (54), Ranunculaceae (37), Brassicaceae (21), and Orobanchaceae (21) having the largest number of seed samples (Figure 1). Among the cryopreserved 194 species of herbaceous plants, 116 are under protection according to the legal regulation, of which 20 species are under partial protection (Table S1). As there is no published method for the freezing of seeds in LN for most herbaceous species, the Cryopreservation Laboratory optimizes the method of drying and cryogenic freezing of genetic resources. Accordingly, the seeds were directly frozen and stored in LN.
Since 2017, Kostrzyca FGB has implemented a project aimed at identifying and storing DNA of rare and endangered plants from the Białowieża Forest area. Seed samples of the most precious species from the area have been routinely stored in cold rooms (at −10 °C and −20 °C) and cryopreserved. In the future, a comparison of the survival rates of these seed samples stored at different temperatures will allow for the selection of optimal long-term storage temperatures for individual species.
All herbaceous plant species have been represented as herbarium specimens. Furthermore, Kostrzyca FGB introduces the barcode sequences of plants to international databases, such as the Barcode of Life Data System (BOLD System). Duplicates in the Kostrzyca FGB collection have been deposited in Powsin (PAS BG-CBDC) and the United Kingdom (Millennium Seed Bank).
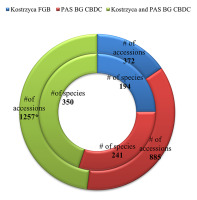
To mitigate the loss of the most important species, the seeds of 85 taxa of high conservation value in Poland were individually cryopreserved at the PAS BG-CBDC and Kostrzyca FGB or duplicated between these institutions. As of January 2022, 1,257 seed accessions of native herbaceous plants were collected across Poland and stored in both cryobanks. The accessions represent a total of 350 species native to Poland (which constitute approximately 13% of the Polish flora) from 51 botanical families (Figure 3). The most represented families were found to be Asteraceae, Fabaceae, Ranunculaceae, Cyperaceae, and Caryophyllaceae. Currently, 195 law-protected species are stored in LN, representing 55.5% of the species protected in Poland. Within this group, 162 species are under strict legal protection while 33 are under partial protection according to the Polish regulation of the Minister of the Environment on October 9, 2014, on the conservation of plant species (Regulation of the Minister of Environment, 2014) (Table S1).
Figure 3
Structure of the cryogenic collection of seeds of herbaceous plants in relation to the storing institutions: Kostrzyca Forest Gene Bank (Kostrzyca FGB) and Polish Academy of Sciences Botanical Garden – Center for Biological Diversity Conservation in Powsin, Warsaw (PAS BG-CBDC) (* including duplicated accessions in both institutions). # – number.
The European Tripartite Garlic Cryobank in the Research Institute of Horticulture – National Research Institute in Skierniewice (InHort)
The InHort cryobank was established in 2011 under the framework of the EURALLIVEG project. The main aim of the project was to establish a European integrated Allium L. core collection based on the national collections of Germany, the Czech Republic, Poland, Italy, France, and Nordic countries. The Cryobanks Network was organized by three project partners: InHort-Poland, Crop Plant Research Gatersleben (IPK) in Germany, and the Crop Research Institute (CRI) in Czech Republic (Keller et al., 2012). Garlic is propagated vegetatively because it does not form fertile seeds. However, field collections of garlic are expensive to manage and susceptible to viral infections and endophytes (Keller et al., 2013). The InHort field collection of garlic (Allium sativium L.) in Skierniewice comprised of 570 accessions in 2017. In the InHort cryobank, 168 garlic accessions from three partner countries were stored (Olas-Sochacka, 2017).
In 2022, the InHort field collection was represented by 754 garlic accessions, including 358 bolting forms, 284 non-bolting forms, and 112 multiannual wild species of the genus Allium L. Among 228 accessions cryopreserved in the cryobank, 143 originated from the InHort collection, 51 from the CRI collection, and 34 from IPK in Germany, with the assumption of a safe duplicate strategy (M. Chojnowski, personal communication, 2022). Both bolting and non-bolting accessions were stored in LN using the vitrification method with PVS2 or PVS3 solution for the dehydration of shoot tips isolated from bulbils (bolting forms) or cloves (non-bolting forms) (Olas-Sochacka, 2017; Olas-Sochacka & Kotlińska, 2015). In most studies, the survival and regeneration rates of non-bolting accessions were higher than those of bolting accessions (Keller et al., 2012; Olas-Sochacka, 2017). All accessions tested in the InHort cryobank exhibited a regeneration rate exceeding 30%, which was sufficient for long-term storage when 100 garlic explants per accession were frozen (Olas-Sochacka, 2017). Each year, ten new accessions are added to the InHort garlic cryobank.
Młochów Research Center of the Plant Breeding and Acclimatization Institute – National Research Institute (PBAI-Młochów RC)
Cryopreserved potato (Solanum tuberosum L.) accessions have been collected since 2005 at the PBAI-Młochów RC as part of the National Center for Plant Genetic Resources. Breeding research and parental line breeding programs for potatoes are supported by the collection of wild and cultivated Solanum L. species and interspecific hybrids. The collection of diploid Solanum L. species includes preserved resources for breeding resistance against viral, bacterial, and fungal pathogens, as well as sources of quality traits. Interspecific hybrids, both at the diploid and tetraploid levels, are potential parental forms for variety breeding (Zimnoch-Guzowska & Flis, 2021).
Tetraploid hybrids are maintained in the field and in in vitro collections, while unique diploid forms are additionally cryopreserved using shoot tip vitrification and PVS2 dehydration (Kryszczuk et al., 2006; Smyda-Dajmund, 2017). Currently, 56 outstanding diploid interspecific hybrids have been cryopreserved as shoot tips. The rate of meristem regeneration varies among the genotypes from 10% to 70%, with an average of 38.5%. Pollen grains of 96 diploid hybrids, 19 diploid wild Solanum L. species, and 17 tetraploid Solanum tuberosum L., including varieties and breeding lines, were cryopreserved by direct freezing in LN. Pollen is conserved by direct immersion in LN, and after thawing, it is efficiently used in crossbreeding (Table 1). Data from cryopreserved Solanum L. accessions have been included in the EGISET database since 2010.
. Possibilities for the Long-Term Storage of Genetic Resources of Forest Tree Species
Multiple attempts have been made to conserve tree genetic resources by subjecting seeds or their parts to ultra-low temperatures. The cryopreservation of tree seeds began in the 1970s when the work of Engstrom (1966) on Pinus sp. was published. Later, many other species were included for cryopreservation studies (Gantait et al., 2016). However, existing techniques and strategies for cryopreservation have been continuously improved, and new techniques have been developed.
Currently, cryopreservation is recognized as a major backup in support of classical storage of orthodox, i.e., tolerant to desiccation, seeds in forest gene banks, as well as the main storage method of short-lived orthodox species, including some members of the Salicaceae family (Michalak, Plitta, et al., 2015). Cryopreservation is the only technique currently available that can be applied for the long-term conservation of the germplasm of forest species that do not produce seeds or produce recalcitrant seeds that are sensitive to desiccation. The summary of achievements in the cryopreservation of the forest genetic resources, for which cryotechniques were successfully developed at the Institute of Dendrology PAS in Kórnik, and applied into practice at the Kostrzyca FGB Cryogenic Laboratory (Chmielarz et al., 2011), are presented in the following sections.
Cryopreservation of Orthodox and Suborthodox Seeds
The key problem in approaches to the cryogenic storage of orthodox seeds is determining their optimum (safe) range of moisture content (SRMC). Although lowering the moisture content increases longevity, desiccation below a certain threshold can be counterproductive (Walters, 2015). B. S. P. Wang et al. (1993) suggested that the SRMC of seeds stored in LN needed to maintain their maximum viability is 3.8%–11%, while Stushnoff and Juntilla (1978) reported a range of 5.5%–11%. Because of the observed differences in seed tolerance to desiccation among species, the specific range of seed moisture content should be determined separately for each species (Chmielarz, 2009a, 2009b; 2010a, 2010b, 2010c, 2010d). Therefore, a series of studies conducted on broad-leaved forest tree species aimed to assess the sensitivity of orthodox and suborthodox seeds to severe desiccation and ultra-low temperature.
It was found that cryostorage of seeds was possible for over 20 species of native trees and shrubs growing in Poland. The physiological responses of seeds to severe desiccation and the temperature of LN by specifying the critical moisture content and high moisture freezing limit (HMFL) for individual species were described. Moreover, it was also proved that the orthodox seeds of different species varied in their response to similar storage conditions (Pritchard, 2007). Orthodox seeds differ in their sensitivity to strong desiccation (5%, 8%, and 11%) (Walters et al., 2004) and storage temperature (−3 °C, −18 °C, or −196 °C). This previous study also observed a significant interaction between seed moisture content and storage temperature for up to four winters. These findings allowed for the development of recommendations on the safe storage of seeds of the selected species for forest practice, primarily for forest nursery production and gene banks (Wawrzyniak, Jasińska, et al., 2020; Wawrzyniak, Kalemba, et al., 2020; Wawrzyniak, Michalak, & Chmielarz, 2020). A summary of the findings achieved at the Institute of Dendrology PAS in Kórnik is presented below.
Seeds of common beech (Fagus sylvatica L.) are of the suborthodox category and abundant in lipids. Therefore, their traditional storage at optimal temperatures (from −3 °C to −10 °C) is limited to several years because they lose their germination capacity. A SRMC has been proposed for these seeds (moisture content of unstratified nuts before cryostorage at the level of 9%–13% or of stratified nuts at 9%–15%), which protects the seeds during cryopreservation in LN and greatly extends the potential time of their storage (Chmielarz, 2007). For other species belonging to the suborthodox category, such as black poplar, an SRMC in the range of 7%–17% was determined (Michalak, Plitta, et al., 2015).
The dieback of common ash (Fraxinus excelsior L.), caused by the sexual stage of the fungus Hymenoscyphus fraxineus (T. Kowalski) Baral, Queloz & Hosoya, is a destructive disease that leads to the high mortality of trees in Poland as well as in other European countries (Klesse et al., 2021; Stroheker et al., 2021). The cryostorage of dormant seeds of this tree species in gene banks is possible (Chmielarz, 2009a). Although classified by most forest researchers and practitioners as tolerant to strong desiccation, ash seeds do not tolerate reduction to a moisture content of 5% (or lower), which is the threshold value for classifying seeds as orthodox (Roberts, 1973). This was reflected in the strongly reduced seedling emergence rates of severely desiccated seeds, whereas the germination rate was at the same level as that of non-desiccated seeds. The SRMC for F. excelsior was found to be 7.2%–19.5%. Similar desiccation behavior was observed for the seeds of Malus sylvestris (L.) Mill, which are also characterized as orthodox but do not tolerate desiccation below 6% moisture content. The SRMC for M. sylvestris seeds was determined as 6.2%–19.4% (Michalak, Plitta-Michalak, & Chmielarz, 2015).
Dormant seeds of the common hazel (Corylus avellana L.) tolerate severe desiccation. However, despite their tolerance to low moisture content, they only tolerate LN within a very narrow range of MC (8%–10%). This is a rare and interesting response of orthodox seeds, as tolerance to desiccation usually ensures the successful cryopreservation of seeds (Michalak, Plitta, & Chmielarz, 2013). A similar response as of orthodox seeds has been observed in wild cherry (Prunus avium L.) (Chmielarz, 2009b) or Prunus padus seeds (Popova et al., 2016). Seeds of these species have a high lipid content, which can cause a specific response to cryogenic storage. Finally, SRMC was determined for other seeds of broad-leaved trees desiccated and stored in LN, such as small-leaved linden (Tilia cordata Mill.) 5.2%–20.1% (Chmielarz, 2002), wild cherry (Prunus avium L.) 9.0%–16.9% (Chmielarz, 2009b), black alder [Alnus glutinosa (L.) Gaertn.] 2.7%–19.2% (Chmielarz, 2010a), silver birch (Betula pendula Roth) 2.0%–23.2% (Chmielarz, 2010b), mountain elm (Ulmus glabra Huds.) 3.3%–17.7% (Chmielarz, 2010c), common hornbeam (Carpinus betulus L.) 3.2%–16.5% (Chmielarz, 2010d), and Caucasian wingnut [Pterocarya fraxinifolia (Lamb.) Spach] 2.8%–18.1% (Wawrzyniak, Jasińska, et al., 2020). For desiccated seeds, the cryogenic temperatures did not reduce the germination capacity of LN-recovered seeds. Only with wild cherry, the emergence obtained from seeds stored in LN was slightly lower compared to the control (non-frozen seeds) (Chmielarz, 2009b).
In summary, recent studies on the cryopreservation of orthodox and suborthodox seeds have shown that seeds rich in lipids have a narrower SRMC.
Cryopreservation of Recalcitrant Seeds
Recalcitrant seeds do not tolerate desiccation below a relatively high level of moisture content (Roberts, 1973) and comprise seeds of approximately 33% of known tree species (Wyse et al., 2018). The level of this “recalcitrance” depends on the species and climatic conditions during seed development (Daws et al., 2006). During their collection, mature recalcitrant seeds still contain too much water to be cryopreserved without ice crystallization, which causes lethal damage to the tissue. The main attributes of recalcitrant seeds that need to be considered before cryopreservation are the critical water content, below which sensitive tissue dies, and the size of the selected tissue. During cryopreservation, desiccation-rehydration and cooling-warming must occur very quickly, and the lowest possible level of moisture content of the preserved tissue must be reached. Tissue size affects the rate of dehydration, cooling, and cryoprotectant penetration, especially of very small samples of material (<1 mg dry mass). Therefore, for the successful cryopreservation of recalcitrant seeds, small parts of the tissue should also be more tolerant to desiccation (mostly embryonic axes or plumules) (Chmielarz et al., 2011). One of the most successful cryopreservation procedures for isolated plant tissues, such as embryonic axes, plumules, shoot tips, embryogenic tissue (Barra-Jiménez et al., 2015; Martı́nez et al., 2003) or dormant buds (Bonnart et al., 2014), is vitrification (Derreudre et al., 1990; Walters et al., 2008). Vitrification can be achieved through a preculture of excised meristems on solidified medium with 0.3 M sucrose or special treatment (osmoprotection) in a vitrification solution (glycerol, sucrose solutions, PVS2, or PVS3). Rapid cooling in LN and rapid warming have also been applied to facilitate vitrification (Niino et al., 1992; Paulus et al., 1993). After thawing, the material must be treated with different sucrose (0.3–1.2 M) or sorbitol (0.6–1.8 M) solutions (unloading) to prevent the meristems from osmotic shock. In vitro culture is used to regenerate whole plants from cryopreserved explants (Chmielarz et al., 2005, 2011). It is important to optimize the time (30–50 min), concentration (0.4–2 M), and temperature (0–25 °C) of each exposure in every step of meristem treatment of individual species. Vitrification has been successfully applied to cold-acclimated and precultured woody plant meristems. Examples of tree species whose germplasm-embryonic axes (EA), shoot tips (ST), somatic embryos (SE), plumules (P), or embryogenic culture (EC) have been successful cryopreserved using vitrification techniques, with their survival or recovery after thawing being 50%–80%, include Artocarpus heterophyllus Lam. (EA) (Thammasiri, 1999), Castanea sativa Mill. (SE) (Corredoira et al., 2004), Malus spp. (ST) (Wu et al., 2001), Morus bombycis Poir. (ST) (Niino et al., 1992), Populus alba L. (ST) (Lambardi et al., 2000), Prunus domestica L. (ST) (De Carlo et al., 2000), Quercus suber L. (SE) (Valladares et al., 2004), Q. robur L. (P) (Chmielarz et al., 2011), Q. robur L. (EC) (Chmielarz, 1999), and Q. robur L. (EA) (Chmielarz, 1997).
Dehydration and cryopreservation of desiccation-sensitive seeds or their fragments must be rapidly after harvest. While seeds collected from tropical areas should be preserved within several days or weeks, recalcitrant seeds collected from temperate regions should be performed for cryopreservation after several weeks or months (Chmielarz et al., 2011). The potential to cryopreserve embryonic axes isolated from many recalcitrant seeds is limited by the damage induced during desiccation. But desiccation is necessary to protect tissue against cellular ice (re-)crystallization during cooling and rewarming. Rapid dehydration lowers the critical water content for the survival of Quercus robur embryonic axes or plumules (Chmielarz et al., 2011) and reduces the accumulation of damage resulting from desiccation-induced aqueous-based deleterious reactions (Ntuli et al., 2011).
Cryopreservation of Embryonic Axes
If whole seeds do not tolerate LN storage, embryonic axes can be used (Kovalchuk et al., 2014). To determine the feasibility of cryopreservation of the embryonic axes of six oak species (white oaks: Quercus macrocarpa Michx., Q. robur, and Q. virginiana Mill. and black oaks: Quercus kelloggii Newb., Q. shumardii Buckley, and Q. velutina Lam.), their sensitivity to desiccation and lethal ice crystallization was investigated (Chmielarz & Walters, 2007). Significant differences were observed between black and white oaks at the physiological level. The embryonic axes of black oaks lost water during desiccation faster than those of white oaks and tolerated drying to a lower level of moisture content (approximately 20% of the fresh seed weight) than those of white oaks (approximately 30%) (Chmielarz & Walters, 2007).
Based on differential scanning calorimetry (DSC), it has been proved that during the cooling of oak embryonic axes, to avoid the lethal ice crystallization within cells, the embryonic axes of black oaks must be dried to a moisture content of 30%. In contrast, to avoid ice crystallization in the embryonic axes of white oaks, they must be dried to a lower moisture content (20%–24%), which is harmful because of excessive desiccation, as shown during in vitro growth. Thus, it can be concluded that the successful cryostorage of the embryonic axes of Q. robur is not possible without the use of cryoprotectants (Chmielarz & Walters, 2007; Walters et al., 2008). However, it is worth noting that the cryopreservation of Quercus robur (Berjak et al., 1999; Chmielarz, 1997) and Fagus sylvatica L. (Nuc et al., 2016) embryonic axes has been partly successful using vitrification techniques.
Cryopreservation of Quercus robur Plumules
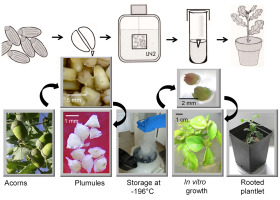
A plumule is an apical meristem (apical dome with three or four leaf primordia) that is isolated from the embryonic axis. For the cryopreservation of the Q. robur germplasm, plumules can be more promising explants than whole embryonic axes or dormant buds (Choudhary et al., 2014) This is mostly because of the homogeneous structure of the plumule tissue in comparison with the structure of an embryonic axis, especially a radicle. Regarding plumule cryopreservation, approximately 60% of plumules were found to survive storage in LN, whereas 25% of regenerated shoots could be rooted (Figure 4). This experiment was repeated several times on acorns of different provenances, with positive results both in Kórnik and Kostrzyca FGB (Chmielarz et al., 2011). This cryopreservation technique has been applied to oaks in the Kostrzyca FGB.
Figure 4
Cryopreservation of Quercus robur plumules. Left to right: Acorns (bottom) from which embryonic axes (EA) were isolated (top); plumules isolated from EA (bottom); Dewar tank for LN storage; plumules recovered in vitro after LN storage (top); leafy shoots developing from plumules and rooted plantlet (6-month-old) from cryopreservation-derived plumules (bottom).
The successful preservation of biological material in gene banks, as well as in LN, requires the conserved material to maintain stability at the structural, physiological, biochemical, genetic (DNA sequence), and epigenetic (cytosine methylation, modifications of histones) level. Research has attempted to explain the potential changes that occur in the cells during seed desiccation and storage at each of these levels (Plitta-Michalak et al., 2021). Plants can modulate their physiology through genome-wide changes in gene expression driven by epigenetic processes in response to environmental conditions, including severe desiccation (Plitta-Michalak et al., 2018). Therefore, analyzing DNA methylation (5-methylcytosine), which plays an important role in the response to external stimuli, is of great interest. Such changes were investigated using two-dimensional thin-layer chromatography (2D TLC) for Pyrus communis L. orthodox seeds and seedlings after seed desiccation and storage (Michalak, Barciszewska, et al., 2013). As a result, the changes in the overall m5C content of the DNA of Quercus robur plumules during the successive stages of their pretreatment before cryopreservation (with cryoprotectants and drying) and after storage in LN were found to be small. Thus, it can be assumed that this procedure is safe (Plitta et al., 2014). This was confirmed by the results of the plumule viability assessment after storage in LN, where the survival rate was maintained at a relatively high level of approximately 60%.
Restoration of Monumental Oaks Growing in Poland
The micropropagation of oaks (Quercus robur L.) has been previously described for younger trees (30 to 50 years) (Chalupa, 1988, 1993). However, there are no reports of the successful in vitro culture of 500- to 800-year-old oaks. The in vitro cloning of the oldest monumental oaks growing in Poland was the largest attempt to use the experience of in vitro culture of Q. robur (Figure 5). In studies on 20 monumental oaks from different sites in Poland, researchers found that 500- to 800-year-old oaks showed much lower potential for shoot multiplication when cultured in vitro compared to 70-year-old oaks. Among the 800-year-old oaks, some multiplied and rooted in vitro very well, similar to the younger (70-year-old) oaks. Clones of selected monumental oaks maintained high growth rates after their transfer to in vivo conditions in a solid medium (Kotlarski et al., 2019). The genetic identity of the cloned tree of Rus oak from Rogalin with the maternal tree was confirmed using simple sequence repeat (SSR) microsatellite markers.
Research on the cloning of monumental oak trees closes, for now, the series of studies concerning the preservation of genetic resources of Q. robur ex situ, including experiments evaluating the cryopreservation of embryonic axes, embryogenic tissue, and plumules.
Figure 5
(A) 800-year-old oak Rus (Quercus robur L.) growing in Rogalin (photograph: Tomasz Siuda). (B) Fragments of woody shoots collected from the old tree in late April 2014. Samples were kept in water-filled containers in a growth chamber with high levels of humidity at 25 °C for the preparation of explants. (C) Young epicormic shoots developed from sleeping buds of woody branches (1-month-old explants). (D) Disinfection of 2 cm-long fragments of epicormics shoots, from which leaves were removed (explants for initiation of in vitro culture). (E) Sterile shoots with one–two buds cultured in vitro on woody plant medium containing cytokinins (6-benzylaminopurine) (initiation of in vitro culture). (F) Multiplication of shoots in sterile conditions, in light, at 23 °C. (G) Rooted shoots and rooting medium with activated carbon [rooting of shoots (minimum 2.5 cm in high) in vitro]. (H) Acclimated plantlets growing in high containers with solid medium, with new leaves visible. (I) The clone of oak Rus derived in vitro (the same genotype of root and shoot), planted into a 120-litre container. (J) The National Museum in Poznań – Rogalin Palace, where the clone of oak Rus was planted in April 2019. (K) A 4-year-old oak tree, a clone of oak Rus. (M) The first acorns appeared on the tree very early (in 2021), serving as a reminder of the real genetic age of the tree.

. Final Remarks
In Poland, the efficient use of LN at cryobanks of PAS BG-CBDC and Kostrzyca FGB for long-term storage of seeds of a wide range of rare and endangered plant species and forest trees helps create a safe backup storage system for the genetic diversity of this group of plants. The cryopreservation of selected genotypes of vegetatively reproduced species (e.g., apple, garlic, and potato) has helped secure a growing number of unique forms of these species. Freezing pollen grains of potential parental forms in LN provides excellent protection of their gene pool for further use in breeding work. Thus, LN treatment is recommended for use in the breeding of numerous plant species. Many years of research by several groups of scientists from the Institute of Dendrology has greatly enriched our knowledge in the field of the cryopreservation of organs and parts of trees and shrubs, as well as expanded research activity into the field of collecting seeds and tissues of Polish and world forest gene banks. Ferns, as a hitherto poorly known group of plants, should be studied more intensively in the future to provide a basis for the effective protection and rational utilization of their biodiversity.
. Supplementary Material
The following supplementary material is available for this article:
Table S1. The number of seed accessions of herbaceous taxa cryopreserved in two cryobanks in Poland: Polish Academy of Sciences Botanical Garden – Center for Biological Diversity Conservation in Powsin, Warsaw and the Kostrzyca Forest Gene Bank; as of the January 2022.
Table S2. The collection of 289 apple varieties stored in vapor liquid nitrogen in Polish Academy of Sciences Botanical Garden – Center for Biological Diversity Conservation in Powsin, Warsaw.
Table S3. Ferns stored as gametophytes in cryobanks worldwide.


