Introduction
The genus Trentepohlia Martius, a subaerial or terrestrial alga, is the largest genus in the family Trentepohliaceae, order Trentepohliales, class Ulvophyceae and division Chlorophyta (Allali,2011; Guiry & Guiry,2015; John,2002; López-Bautista et al.,2002; Rindi,2007; Rindi et al.,2009). The genus has two forms: prostrate filaments and erect filaments (John,2002; López-Bautista et al.,2002; Rindi,2007; Rindi et al.,2009). The algae grow on a wide range of substrate, ranging from natural ones such as rocks, bark, stems, leaves, and the fruits of trees, to various manmade materials; they also grow in close association with fungi, forming lichens (Allali,2011; Guiry & Guiry,2015; John,2002,2003; López-Bautista et al.,2002; Rindi,2007; Rindi et al.,2009). Trentepohlia is the most diverse and dominant of the subaerial algae found in tropical and subtropical regions (Allali,2011; John,2002; López-Bautista et al.,2002; Rindi,2007; Rindi et al.,2009). In addition, all Trentepohlia species produce large amounts of carotenoids, which protect them from ultraviolet (UV) light and high irradiance. Their pigments give them a yellow, orange, or red color that is easily spotted in natural habitats (López-Bautista,2008). Among the carotenoids found in Trentepohlia, β-carotene is the commonest one, followed by zeaxanthin, neoxanthin, lutein, ascorbic acid, and α-tocopherol (Abe et al.,1998,1999; Aburai et al.,2013; Chen et al.,2015; Czeczuga & Maximov,1996; Mukherjee et al.,2010).
These subaerial algae have been little studied, and not much is known about their biodiversity, ecological features, and taxonomy. As currently defined, the genus Trentepohlia contains 52 species and 21 varieties (Allali,2011; Guiry & Guiry,2015; López-Bautista,2008; López-Bautista et al.,2002). Previous studies of Trentepohlia have mainly focused on species from Europe, Central America, South America, Africa, and some countries in Asia (Allali,2011). Only a few studies have been carried out in important tropical and subtropical regions. During the last two decades, most of the new subaerial algae have been found in the tropical zone (Allali,2011). In Thailand, a country in the tropical zone of Asia, no research has been done directly on the ecology, diversity, and distribution of this subaerial alga (Lewmanomont et al.,1995). The natural accumulation of β-carotene in Trentepohlia can be so plentiful that it may be possible to utilize it in a number of commercial products (López-Bautista,2008; Rindi et al.,2009). Some scientists have suggested that these algae could be used as a rich source of food for humans, natural food colorants, animal feed, cosmetics, and medicine (e.g., a source of vitamin A, an enhancer of the immune response, an antioxidant or an anticancer drug) (Cazzonelli,2011; Eldahshan & Singab,2013; Guedes et al.,2011; Mortensen,2006; Priyadarshani & Rath,2012; Rao & Rao,2007; Takaichi,2011). Scientists around the world have been trying to collect, extract, and isolate β-carotene from Trentepohlia (Aburai et al.,2013; Chen et al.,2015), but no one has done it yet in Thailand. Kharkongor and Ramanujam (2015) and Mukherjee et al. (2010) reported that β-carotene content could vary in species across different seasons because of the different environmental factors.
We chose the Chiang Dao Wildlife Sanctuary in Chiang Mai Province in northern Thailand as a study site, because within it is the third highest mountain and the highest limestone mountain in Thailand, and it also has forests that are home to rare and endemic species of plants. This is important because Trentepohlia grow in various types of forest and substrata. There has been no research focused directly on the ecology, taxonomy, and β-carotene content of these subaerial algae. Therefore, to carry out such a study, one must first collect preliminary data on the species’ biodiversity. Such data are important for the discovery of new species and for evaluating how environmental factors affect the species. In addition, we were interested in focusing on the extraction and isolation of chlorophyll a and b, total carotenoids, and β-carotene in these species. We also compared the ratios of these pigments in the dominant Trentepohlia species found in various seasons in this important natural habitat in Thailand.
Our study aimed to understand which environmental factors might influence the diversity of algae and pigments in the subaerial green algal genus Trentepohlia in the Chiang Dao Wildlife Sanctuary by surveying and collecting algae in three different seasons. We also studied and compared the amounts of chlorophyll a and b, total carotenoids, and β-carotene in the dominant Trentepohlia species by collecting the algae in season and using HPLC methods to detect and record the phytochemical substances.
Material and Methods
Study Site and Sampling Area
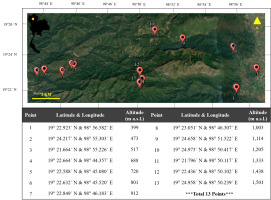
The study sites were located in the Chiang Dao Wildlife Sanctuary in Chiang Mai Province in northern Thailand (latitude 19° 34′ to 19° 62′ north and longitude 98° 64′ to 98° 95′ east), at altitudes ranging from 300 to 2,175 meters above sea level (m a.s.l). The sanctuary has a tropical savanna climate, with an average total rainfall of more than 1,500 mm. Winter is cold, and summer is usually hot and dry. The three main seasons of the sanctuary are the rainy, winter, and summer seasons, each with different rainfall and temperature. The rainy season usually lasts from June to September, with monsoons arriving on June 1st and rains continuing almost until the end of September (19.05 ± 0.15 °C – 30.01 ± 0.45 °C, rainfall 1,200.43 ± 15.01 mm). October 1st till November 30th is the transitional months between the rainy and winter seasons. The winter season lasts from December 1st to February 28th and is characterized by cold weather (15.15 ± 1.50 °C – 24.11 ± 1.63 °C, rainfall 360.65 ± 22.49 mm). The summer season starts after the end of the winter season (March 1st) and lasts until May 31st; it is hot and dry and high light intensity (16.85 ± 1.51 °C – 35.71 ± 1.29 °C, rainfall 201.63 ± 49.78 mm) (Chiang Dao Wildlife Research Station,2018).
Thirteen sampling points were randomly selected at 100-m intervals starting at 300 m a.s.l. and ending at 1,500 m a.s.l. along two trails in the sanctuary, the Ban Yang Thung Pong and Sop Hui Pha Tang Na Lao trails. Their geographical locations and altitudes were marked with the Garmin eTrex 30x handheld GPS unit (Garmin International, United States) (Figure 1). The studies were undertaken in August 2016 (rainy season), December 2016 (winter season), and April 2017 (summer season).
Analysis of Diversity and Environmental Factors
Algal Sampling
Algae were randomly collected with a sterile scraper or a knife from different natural and artificial substrata and placed into plastic boxes for further identification. At each sampling point, some ecological factors, such as the type of substrate, the color of the sample, and preliminary morphological characteristics, were observed and recorded to help with identification.
Measuring Environmental Factors
Environmental factors such as the relative humidity (%), temperature (°C), and light intensity (µmol photon m−2 s−1) were measured in situ from 8.00 a.m. to 4.00 p.m. using the Environment Meter STANDARD: ST-8820 model (Shenzhen Everbest Machinery Industry, China).
Algal Identification
The collected algal samples were kept in the refrigerator at 5 °C in the laboratory. Identification of Trentepohlia species was performed with a stereomicroscope, Olympus SZ30, and a light compound microscope, Olympus CH30 (both from the Olympus Corp., Japan), within 2 weeks after collection to observe more detailed morphologies. Photos were taken with the digital Dino-Eye microscope camera AM423X (AnMo Electronics, Taiwan) and transferred to a computer for further processing with Microsoft Paint (Microsoft Corporation, United States). Scanning electron microscopy was carried out using the Quanta 450 FEI model (Thermo Fisher Scientific, United States) within 1 month after collection to observe the small details of the morphology. Finally, the algae were identified using the relevant floristic keys by Guiry and Guiry (2015), John (2002,2003), and López-Bautista et al. (2002).
Analysis of Similarities Among Algae
Cluster analysis using the Bray–Curtis method was used to determine the similarities among Trentepohlia species in the 13 sampling sites. Some environmental factors were added to determine the similarities using canonical correlation analysis (CCA). The PC-ORDTM 6 program was used for this (MjM Software Design, United States) (Clarke & Warwick,1994). Moreover, a program to measure the Shannon–Weaver diversity index based on species diversity and richness (Pisces Conservation, United Kingdom) was used to compare the diversity of algae in the various habitats of Trentepohlia spp. (Clarke & Warwick,1994).
Analysis of Diversity of Algae and Environmental Factors
The trends in the relationship between algae and certain environmental factors, such as altitude, relative humidity, temperature, and light intensity, were analyzed using a principle correspondence analysis (PCA) with the Bray–Curtis distance set in the PC-ORDTM 6 program. The statistical values of the binary logistic (B) in the statistical program also analyzed the relationships between algae and certain environmental factors (Clarke & Warwick,1994; McCune,2007).
Analysis of Photosynthetic Pigment Content
Algae Materials
The dominant species in this study, T. rigidula (J. Müller) Hariot, was collected from electricity posts in a dry evergreen forest. The largest amount of algae with the brightest color was found at three sampling points at different altitudes. These were Sampling Point 2, the Chiang Dao Wildlife Research Office (473 m a.s.l.), Sampling Point 3, Chiang Dao Wildlife Sanctuary Office (517 m a.s.l.), and Sampling Point 4, Huai Forest Protection Unit (688 m a.s.l.) along the Ban Yang Thung Pong and Sop Hui Pha Tang Na Lao trails, as shown in Figure 1, and were marked by a GPS.
Chemicals and Solvents for Analysis
Analytical reagent grade dimethyl sulfoxide (DMSO) (Fisher Scientific, United Kingdom), and standard ≥95%, synthetic, type II, of β-carotene HPLC grade (Sigma-Aldrich, United States) were used. All the chemicals and solvents were of HPLC grade, such as dichloromethane (Merck, Germany), methanol (Merck), and acetonitrile (RCI Labscan, Thailand). Ultrapure-grade water was obtained from the Scientific Equipment Center, Faculty of Science, Kasetsart University.
Analysis of Total Chlorophyll (a and b) and Total Carotenoid Content
The total chlorophyll (a and b) content and total carotenoid content were measured using the spectrophotometer methods of Chen et al. (2015,2016) and Pompelli et al. (2012), which were modified versions of the Wellburn method (1994). A sample of 10 mg of algae was incubated with 10 mL of DMSO and spun with a vortex, the Fisher Scientific Touch Mixer Model 232 (Fisher Scientific, United States). The samples were kept in a dark room at room temperature for 24 hours and were then centrifuged by the Sanyo MSE Centaur 2 centrifuge (Sanyo, United Kingdom) at 1,339.06 g for 5 minutes and filtered with Whatman filter paper No. 1 (GE Healthcare, United Kingdom). Finally, the absorbance of 5 mL of the supernatant liquid was measured with the T60-Visible spectrophotometer (PG Instruments, United Kingdom) with DMSO as a blank at 480, 649, and 665 nm. The pigment contents were calculated as follows (Pompelli et al.,2012):
These measurements were replicated three times for each site, and the results presented were the means of the three replications. Duncan’s new multiple range test (DMRT) (ANOVA, α = 0.05) was used to find the differences in total chlorophyll (a and b) and total carotenoids during the rainy, winter, and summer seasons.
Analysis of β-Carotene Content
β-Carotene content in the pigment extract was determined using a HPLC analysis method modified from Aburai et al. (2013) and was performed using a reversed-phase column of the Agilent 1100 Series, ChromSpher Si 5 C18 column, 150 mm × 4.6 mm, and a pump from the Agilent 1100 Series equipped with a UV/Vis photodiode detector (all from Agilent Technologies, United States). After grinding the lyophilized sample of 100 mg in an agate mortar for 5 minutes, the algal sample was extracted from the powdered sample using dichloromethane:methanol (DCM) (25:75, v/v) for 24 hours. The sample extract was then dried using the Buchi R-215 Rotavapor (BÜCHI Labortechnik, Switzerland), dissolved in a methanol solution diluted 10 times, and kept in the dark at −20 °C. The aliquots of 20 μL of the lipophilic part were used for the HPLC analysis. The mobile phase consisted of eluent A (dichloromethane:methanol:acetonitrile:water = 25:425:27.5:50, v/v) and eluent B (dichloromethane:methanol:acetonitrile:water = 125:140:212.5:15, v/v). The following elution gradient was used: 0% B for 5 minutes and then a linear gradient from 0% to 60% for 7 minutes; 60% B for 8 minutes and then a linear gradient from 60% to 100% for 20 minutes; and 100% B for 20 minutes and then a linear gradient from 100% to 0% for 5 minutes. During analysis, the flow rate was maintained at 1.0 mL min−1, the column temperature was 50 °C, and the absorbance was monitored at 480 nm. The commercial β-carotene of different concentrations were used as the standard.
The identification of β-carotene in T. rigidula was carried out by comparing the retention time and absorption spectra of unknown peaks to the β-carotene standard, and the quantity of β-carotene was estimated by looking at the chromatogram peak areas and comparing the β-carotene standard curves in various concentrations.
Results
Environmental Factors
Environmental factors in the Chiang Dao Wildlife Sanctuary, Chiang Mai Province, are presented in Figure 2. The relative humidity was high in the winter and rainy seasons, at more than 75%, but was quite low in the summer season, at 52.27%. This finding was the opposite of the data on temperature and light intensity (Figure 2A). Temperature readings were highest in the summer (29.68 °C), followed by the rainy season (27.63 °C), and the winter season (21.85 °C) (Figure 2B). The light intensity values in the summer, rainy, and winter seasons were 248.39 ± 8.42 µmol photon m−2 s−1, 110.17 ± 9.92 µmol photon m−2 s−1, and 47.70 ± 2.40 µmol photon m−2 s−1, respectively (Figure 2C).
Diversity of Algae
Thirteen species of the subaerial green algae Trentepohlia were found in the Chiang Dao Wildlife Sanctuary, Chiang Mai Province (Table 1), including T. chapmanii Rindi & J. López-Bautista (Figure 3A–D), Trentepohlia sp. 1 (Figure 3E–H), Trentepohlia sp. 2 (Figure 3I–L), T. sundarbanensis G. G. Satpati & R. Pal (Figure 3M–P), Trentepohlia sp. 3 (Figure 4A–D), T. rigidula (J. Müller) Hariot (Figure 4E–H), Trentepohlia sp. 4 (Figure 4I–L), T. effusa (Krempelhuber) Hariot (Figure 4M–P), T. monilia (J. Müller) Hariot (Figure 5A–D), T. abietina (Flotow ex Kützing) Hansgirg (Figure 5E–H), Trentepohlia sp. 5 (Figure 5I–L), T. aurea (Linnaeus) C. Martius (Figure 5M–P), and T. umbrina (Kützing) Bornet (Figure 6A–D).
Table 1
Diversity and composition of the subaerial green algae genus Trentepohlia at 13 sampling points during the rainy, winter, and summer seasons.
Figure 3
Subaerial green algae genus Trentepohlia at 13 sampling points in the rainy, winter, and summer seasons, including T. chapmanii (A–D), Trentepohlia sp. 1 (E–H), Trentepohlia sp. 2 (I–L), and T. sundarbanensis (M–P). Scale bars: 20 µm.

Figure 4
Subaerial green algae genus Trentepohlia at 13 sampling points in the rainy, winter, and summer seasons, including Trentepohlia sp. 3 (A–D), T. rigidula (E–H), Trentepohlia sp. 4 (I–L), and T. effusa (M–P). Scale bars: 20 µm.

Figure 5
Subaerial green algae genus Trentepohlia at 13 sampling points in the rainy, winter, and summer seasons, including T. monilia (A–D), T. abietina (E–H), Trentepohlia sp. 5 (I–L), and T. aurea (M–P). Scale bars: 20 µm.

Figure 6
Subaerial green algae genus Trentepohlia at 13 sampling points in the rainy, winter, and summer seasons, including T. umbrina (A–D). Scale bars: 20 µm.
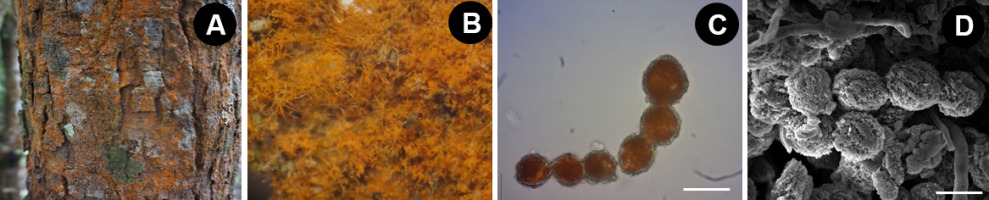
After studying 13 Trentepohlia species, we found that the key morphological characteristics of the species are as follows:
1. Cylindrical cells in the filaments
– 2. Acute or pointed terminal cells
– – 3. Long filaments and a narrow area from the substrate cell part to the terminal cell in the erect part – Trentepohlia effusa (Figure 4M–P)
– – 3. Short filaments and deep attachments to the substrata
– – – – 4. Unbranched filaments – Trentepohlia sp. 4 (Figure 4I–L)
– – – – 4. Branched filaments – Trentepohlia chapmanii (Figure 3A–D)
– 2. Blunt on terminal cells
– – – – 5. Unbranched filaments – Trentepohlia sp. 1 (Figure 3E–H)
– – – – 5. Branched filaments
– – – – – 6. Small cells; the filaments in erect cells stand upright – Trentepohlia sundarbanensis (Figure 3M–P)
– – – – – 6. Large cells; the main filament is curved
– – – – – – 7. Densely branched filaments; the main filament is curved and has large green cells – Trentepohlia sp. 2 (Figure 3I–L)
– – – – – – 7. Sparingly branched filaments; the main filament is straight and has orange cells – Trentepohlia aurea (Figure 5M–P)
1. Global, subglobal or barrel-shaped cells in filaments, with constrictions between the cells
– – – – – – 8. In the filaments, one side of each barrel-shaped cell is swollen – Trentepohlia sp. 5 (Figure 5I–L)
– – – – – – 8. Global, subglobal or barrel-shaped cells in filaments
– – – – – – – 9. Cut terminal cells with an apical cap of pectin – Trentepohlia abietina (Figure 5E–H)
– – – – – – – 9. Blunt terminal cells
– – – – – – – – 10. Rough cell wall – Trentepohlia umbrina (Figure 6A–D)
– – – – – – – – 10. Smooth cell wall
– – – – – – – – – 11. Small cells; growth occurred only on objects made of steel – Trentepohlia sp. 3 (Figure 4A–D)
– – – – – – – – – 11. Small cells; growth occurred on a cement electrical post
– – – – – – – – – – 12. Densely branched filaments – Trentepohlia rigidula (Figure 4E–H)
– – – – – – – – – – 12. Sparingly branched filaments – Trentepohlia monilia (Figure 5A–D)
The morphological characteristics of Trentepohlia sp. 1, Trentepohlia sp. 2, Trentepohlia sp. 3, Trentepohlia sp. 4, and Trentepohlia sp. 5 were unlike any found in the identification keys of the species. Trentepohlia sp. 1 seemed like T. chapmanii but had larger globular cells in erect filaments that were 10.38 to 15.41 µm wide, and 13.69 to 19.48 µm in length and thickness. It also had pectic cells at the tips of the filaments. Trentepohlia sp. 2 was green in color; had large cylindrical cells that were 6.64 to 8.68 µm wide and 24.94 to 28.86 µm long, often slightly pointed at the tip; and had lateral branching. The morphological characteristics of Trentepohlia sp. 3 cells were similar to those of T. rigidula, but sp. 3 had smaller, global, swollen vegetative cells that were 2.67 to 3.59 µm wide and 4.21 to 3.60 µm long, and were often slightly pointed at the tip. Trentepohlia sp. 4 had characteristics that were somewhat like those of T. effusa, but it had shorter filaments (63.08 to 67.51 µm long) and smaller globular cells (3.30 to 4.22 µm wide and 13.15 to 15.82 µm long), with no branching. Finally, Trentepohlia sp. 5 had only reproductive cells with large globular or ovoid zoosporangia on their terminal tips; they were 14.07 to 15.66 µm wide and 15.73 to 20.36 µm long.
At Sampling Points 2 and 3, which were at a low altitude, we found the highest number of species (nine and seven respectively), but only two species were found at Sampling Points 9, 10, 11, and 13 (see Table 1 ). Furthermore, T. chapmanii, T. rigidula, Trentepohlia sp. 1, T. effusa, T. Abietina, and T. umbrina were the species most commonly found at many of the sampling points used during the study (Table 1).
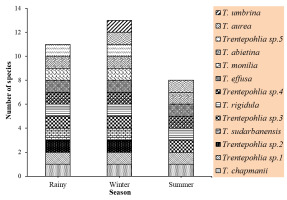
Of the three seasons we studied, we found that the winter season had the highest number of species (13), the rainy season had the next highest number (11), and the summer season had the lowest number (eight) (Figure 7). Moreover, some species were found in all three seasons: T. chapmanii, Trentepohlia sp. 1, Trentepohlia sp. 3, T. rigidula, Trentepohlia sp. 4, T. effusa, and T. abietina.
Figure 7
Diversity and composition of subaerial green algae genus Trentepohlia in the rainy, winter, and summer seasons.
All of the species had many substrates that were attached to a surface. The surfaces were of two types: the bark of trees and manmade constructions (Table 2). Most of the species were found attached both to bark and manmade constructions, including T. chapmanii, Trentepohlia sp. 1, Trentepohlia sp. 3, T. rigidula, Trentepohlia sp. 4, T. effusa, T. monilial, and T. aurea. Species attached only to the bark of various tree species were Trentepohlia sp. 2, T. sundarbanensis, T. abietina, and T. umbrina. One species, Trentepohlia sp. 5, was only found attached to manmade constructions.
Table 2 shows that the highest numbers of species (10) were found in dry evergreen forest areas; these were T. chapmanii, Trentepohlia sp. 1, Trentepohlia sp. 2, T. sundarbanensis, Trentepohlia sp. 3, T. rigidula, Trentepohlia sp. 4, T. effusa, T. monilial, and T. aurea. Nine species were found in planted gardens, six in lower montane rainforest areas, three in deciduous dipterocarp forest areas, two in mixed deciduous forest areas, and two in lower montane coniferous forest areas.
Table 2
Composition of the subaerial green algae genus Trentepohlia on different types of substrate and habitat.
Similarity of Species Composition Among Sampling Sites
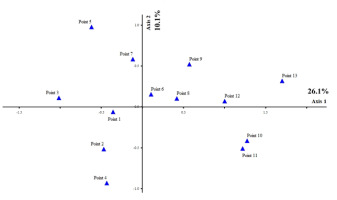
Cluster analysis showed four groups of sampling points (Figure 8) for Trentepohlia, with 75% information remaining. Group 1 (Sampling Points 1, 2, 3, and 4) had the most diversity of algae, with Trentepohlia sp. 1 and T. rigidula as the dominant species. In Group 2 (Sampling Points 6 and 8), T. chapmanii and T. aurea were the dominant species. In Group 3 (Sampling Points 5 and 7), T. chapmanii, T. effusa, and T. monilia were the dominant species. In Group 4 (Sampling Points 9, 10, 11, 12, and 13), there was little diversity of algae, with only T. abietina as the dominant species. Canonical correspondence analysis (CCA) ordination, based on the Trentepohlia species, the sampling sites, and some environmental factors, also showed the results of the four groups similar to the cluster analysis. The sampling points in each group were quite different (26.1%, with 10.1% of the variance explained) (Figure 9). Group 1 used Sampling Points 1, 2, and 4, and had the most diversity of algae; Group 2 used Sampling Points 3, 7, and 5 Group 3 used Sampling Points 6, 8, 9, 12, and 13); and Group 4 used Sampling Points 10 and 11.
Figure 8
Similarity of algal composition by cluster analysis among 13 sampling points in the rainy, winter, and summer seasons for subaerial green algae genus Trentepohlia.
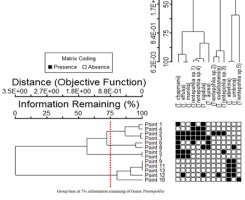
Figure 9
Similarity of algal composition by CCA ordination of the observed variance in environmental factors, across 13 sampling points in the rainy, winter, and summer seasons for subaerial green algae genus Trentepohlia. Axis 1 represents 26.1% and Axis 2 represents10.1% of the observed variance.
According to the Shannon–Weaver diversity index, algal diversity at Sampling Point 2 had the highest diversity index, at 2.20. It was followed by Sampling Point 3 (1.95), Sampling Point 12 (1.61), Sampling Points 5 and 8 (1.38), and Sampling Points 1, 4, 6, and 7 (1.10). The lowest diversity index was found at Sampling Points 9, 10, 11, and 13 (0.69).
Diversity of Algae and the Influence of Environmental Factors
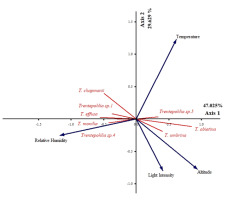
PCA ordination showed the influence of environmental factors on the presence of Trentepohlia species (47.025%, 29.629% of variance explained) (Figure 10). Altitude was a major contributor to the first PCA axis (r = 0.769, r = −0.504). It had a positive influence on T. abietina (r = 0.825, r = −0.307) and T. umbrina (r = 0.568, r = −0.394), but a negative influence on T. chapmanii (r = −0.629, r = 0.563), T. effusa (r = −0.666, r = 0.135), and Trentepohlia sp. 1 (r = −0.544, r = 0.255). Relative humidity was also a major contributor to the first PCA axis (r = −0.960, r = −0.166). This factor had a positive influence on Trentepohlia sp. 4 (r = −0.582, r = −0.223) and T. monilia (r = −0.488, r = −0.061), and a negative influence on Trentepohlia sp. 5 (r = 0.519, r = 0.162). The temperature (r = 0.505, r = 0.792) and light intensity (r = 0.335, r = −0.519) were major contributors to the second PCA axis, and had no influence on the species.
Figure 10
PCA ordination summarizing of the influence of environmental factors on communities of subaerialgreen algae genus Trentepohlia. Axis 1 represents 47.025% and Axis 2 represents 29.629% of the observed variance.
Binary logistic regression showed that all environmental factors had a relationship of some significance (*) with Trentepohlia species, which reflects the PCA ordination analysis results (Table 3). The altitude had a negative impact on the largest number of species, but only for T. chapmanii (−0.007) was the relationship significantly negative. The relative humidity had a positive impact on the largest number of species, and the relationship was significantly positive for T. monilia (0.271). Finally, the temperature had both positive and negative relationships with the algae, and light intensity had the least detectable relationship with the species, but neither of them did not have a significantly positive or negative relationship with any species.
Table 3
Binary logistic regression coefficients of altitude, relative humidity, temperature, and light intensity for the subaerial green algae genus Trentepohlia at 13 sampling points (p ≤ 0.05, n = 36).
Total Chlorophyll (a and b) and Total Carotenoid Content
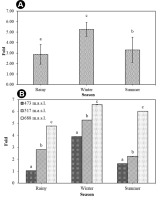
A quantitative estimation of the total chlorophyll (chlorophyll a and b) and total carotenoid content of T. rigidula using the spectrophotometer method showed statistically significant seasonal variations (Figure 11A). The total chlorophyll a and b content in each season varied significantly (p ≤ 0.05, n = 9), from a maximum of 0.36 ± 0.01 %w/w in the rainy season, to a composite content of 0.33 ± 0.01 %w/w in the winter and 0.26 ± 0.01 %w/w in the summer (see Figure 11A). The total carotenoid content in each season (p ≤ 0.05, n = 9) ranged from a maximum in the winter season of 1.64 ± 0.02 %w/w, to a composite content in the rainy and summer seasons of 0.89 ± 0.05 %w/w and 0.76 ± 0.04 %w/w, respectively (see Figure 11B). When considering the ratio of total carotenoid to chlorophyll (p ≤ 0.05, n = 9), we found that the ratio was the highest in the winter, at 4.96:1, followed by the summer season (2.47:1), and rainy season (2.92:1) (Figure 12A).
Figure 11
(A) Total chlorophyll (a and b) and total carotenoid content (%w/w) by season. (B) Total chlorophyll (a and b) (%w/w) by sampling point in each season. (C) Total carotenoid content (%w/w) by each sampling point in each season of T. rigidula collected from electricity posts. Data are the mean values ofthree replications, and the SE is indicated by vertical bars. The different letters above the columns indicate significant differences (p ≤ 0.05, n = 9) at each time by Duncan’s new multiple range test.
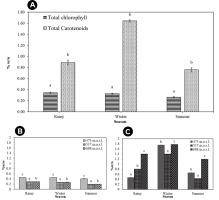
Figure 12
(A) Mean of comparisons of total carotenoid content and total chlorophyll (a and b) ratios and (B) total carotenoid content and total chlorophyll (a and b) ratios in each sampling point in each season of T. rigidula collected from electricity posts. Data are the mean values of three replications, and the SE is indicated by vertical bars. The different letters above the columns indicate significant differences (p ≤ 0.05, n = 9) at each time by Duncan’s new multiple range test.
β-Carotene Content
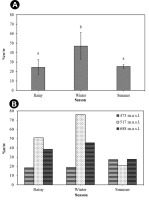
A quantitative estimation of the β-carotene content of T. rigidula using HPLC analysis showed significantly different accumulation rates in different seasons. The β-carotene in the HPLC spectra was identified using different β-carotene content standards as a reference. The standard β-carotene had a retention time of 35 to 39 minutes. On the HPLC chromatograms, only one tall peak was seen for all of the algae samples at 35 to 39 minutes. Therefore, we can conclude that most of the carotenoids were β-carotene. The amount of β-carotene in the algae samples was derived from the area under the peak with reference to different β-carotene content standards (Figure 13). The β-carotene content (%w/w) of T. rigidula showed significant seasonal variation (p ≤ 0.05, n = 3) (Figure 14A). The quantitative amount of β-carotene was highest in the winter season (46.89 ± 14.28 %w/w), followed by the summer season (25.30 ± 1.88 %w/w), and rainy season (24.46 ± 8.18 %w/w) (see Figure 14A).
Figure 13
The example HPLC chromatogram of the β-carotene content of T. rigidula at three sampling points in winter season. Retention time of violaxanthin peak in 3–4 min, canthaxanthin peak in 10–12 min, chlorophyll b peak in19–21 min, and β-carotene peak in 35–39 min.

Figure 14
(A) β-Carotene (%w/w) content by season. The data are the mean values of three replications, and the SE is indicated by vertical bars and the different letters above the columns indicate significant differences (p ≤ 0.05, n = 3) at each time by Duncan’s new multiple range test. (B) β-Carotene (%w/w) content by each sampling point in each season of T. rigidula collected from electricity posts.
Discussion
Algae Diversity
Based on the environmental factors of Chiang Dao Wildlife Sanctuary, Chiang Mai Province, the Climatological Group, Meteorological Development Bureau, Meteorological Department (2015) has labeled the region as a tropical savanna; this is “Aw” according to the Köppen climate classification.
The level of diversity of Trentepohlia species in this study is similar to that in previous studies by Allali (2011) and Allali et al. (2013) in Les Monts de Cristal National Park, Gabon, at an altitude of 100 to 800 m a.s.l., where 11 species were found. Kharkongor and Ramanujam (2014) studied areas of mixed plantation and open disturbed forest in Meghalaya, India, at an altitude of 958 to 1,710 m a.s.l., and found 11 species, and Neustupa and Škaloud (2008) studied the diversity of subaerial algae and cyanobacteria on tree bark in tropical mountain habitats in a Southeast Asian mountain rainforest in the Cibodas Biosphere Reserve, West Java, Indonesia. The results showed that Trentepohliales were the dominant order, particularly T. aurea, T. monilia, and Trentepohlia sp. We found the highest numbers of species at low altitudes than at higher altitudes in study area. In the lowlands, Trentepohlia was the most diverse and dominant of the subaerial algae that are usually found in abundance in tropical and subtropical regions (Allali,2011; John,2002; López-Bautista et al.,2002; Rindi,2007; Rindi et al.,2009). The Chiang Dao Wildlife Sanctuary is covered by a dense, dry evergreen forest in the tropical zone, which has high humidity and low light intensity (Chiang Dao Wildlife Research Station,2018; Wildlife Conservation Office,2015). Therefore, most species of Trentepohlia were found in these areas because they thrive under these conditions. Moreover, T. chapmanii, T. rigidula, Trentepohlia sp. 1, T. effusa, T. abietina, and T. umbrina were the most commonly found species at various sampling points during the study, which fits with the findings of Kharkongor and Ramanujam (2014). They reported a dominance of Trentepohliales members in an open area with low temperatures and low light intensity, especially of T. abietina and T. rigidula. These species normally grow in many places around the world and are commonly found in diverse habitats.
Of the three seasons we studied, we found that the winter season had the highest number of species. Altitudes in the Chiang Dao Wildlife Sanctuary range from 300 to 2,175 m a.s.l., and as seen in Figure 2, the winter is characterized by low light intensity, and the highest air humidity across all three seasons. These factors could be the reason for the enhancement of Trentepohlia growth in winter. A low temperature (25 °C) along with light provided by cool white fluorescent lamps (43.7 µmol photon m−2 s−1) has been reported to induce physiological activity in algal cells such as Dunaliella salina (Dunal) Teodoresco in the laboratory (Pisal & Lele,2005). The lower number of species in the rainy and summer seasons could be attributed to the high amount of rainfall during these seasons. During the four rainy months (June, July, August, and September), the total rainfall was as much as 1,468.56 ± 544.18 mm (Chiang Dao Wildlife Research Station,2018; Wildlife Conservation Office,2015). The constantly damp conditions and the cloudy days might contribute to the low number of species seen in the rainy and summer seasons. Furthermore, some species were found in all three seasons: T. chapmanii, Trentepohlia sp. 1, Trentepohlia sp. 3, T. rigidula, Trentepohlia sp. 4, T. effusa, and T. abietina. These species were found in all three seasons because of species of Trentepohlia can successfully adapt to terrestrial environments. Some characteristics of these algae are found to improve survivability, including the presence of a sporopollen-like compound, the production of β-carotene, and the cell wall (López-Bautista,2008; López-Bautista et al.,2002).
Similarities of algal composition among the sites were measured using cluster analysis and CCA analysis, and results showed that Group 1 had the highest diversity of algae in a low altitude conform with the high Shannon–Weaver diversity index score, with Trentepohlia sp. 1 and T. rigidula as the dominant species. Rindi (2007) and Rindi et al. (2009) noted that the most diverse and dominant of the subaerial green algae Trentepohlia were abundant in tropical and subtropical regions. Sampling Points 1 to 4 were in low-altitude dry evergreen forests, which had high humidity and low light intensity, conditions that favor the growth of Trentepohlia. On the other hand, the other groups and other sampling points had lower species numbers, likely due to unfavorable environmental factors.
Environmental factors had an effect on the presence of Trentepohlia species in these areas, with higher altitude having an especially negative influence on T. Chapmanii, a species mostly found in lowlands. The high relative humidity had a positive influence on T. effusa. Relative humidity is an environmental factor that encourages a high growth rate in Trentepohlia, especially in T. effusa, according to the researchers (Guiry & Guiry,2015; López-Bautista,2008; López-Bautista et al.,2002). López-Bautista (2008), Rindi (2007) and Rindi et al. (2009) stated that Trentepohlia were among the most widespread microorganisms occurring in tropical terrestrial environments and were influenced by specific environmental factors in various substrata, such as the pH, humidity, wind, light intensity, and temperature. It is likely that detailed surveys conducted in other tropical regions will reveal a wider geographical distribution of many other species of Trentepohlia. Rindi (2007) reported that wind and rain are the main agents of dispersal for Trentepohlia. Therefore, the distribution of Trentepohlia are specific to the different climate zones on Earth. Light conditions were found to be a principal factor for influencing the diversity and composition of the species; this was seen mostly in open, nonshaded microhabitats (Neustupa & Škaloud,2008).
Pigment Content Variability
A quantitative estimation and ratio of the total carotenoid and total chlorophyll (chlorophyll a and b) content of T. rigidula showed statistically significant seasonal variation, with the highest values found in the winter season. The ratio of total carotenoids to chlorophyll is normally high for Trentepohlia in a subaerial habitat, because it requires protection from the strong light of the sun. The increased production of total carotenoids at a low temperature and high light intensity has been reported in T. aurea (Abe et al.,1998), T. arborum (Chen et al.,2015), and T. odorata (Tan et al.,1993). Mukherjee et al. (2010) studied two species of Trentepohlia from natural habitats in Assam State, India, and found that the total carotenoid content in both species was very high, with a small amount of chlorophyll. Kharkongor and Ramanujam (2015) studied the accumulation of total carotenoids in four Trentepohlia species in Shillong, India, and found a high accumulation of carotenoids (especially β-carotene) in their filaments.
A quantitative estimation of the β-carotene content of T. rigidula showed a significantly different accumulation rate at different seasons. A significant seasonal variation was also seen for the total carotenoid content, which was the highest in the winter and lower in the summer and rainy seasons. The high content of total carotenoids in winter could be due to light, water, and temperature stresses. The Chiang Dao Wildlife Sanctuary, which encompasses altitudes ranging from 300 to 2,175 m a.s.l., in low altitude of area (473–688 m a.s.l.) had a low light irradiance (88.25 ± 18.86 µmol photon m−2 s−1) during the daytime, along with humidity (85.53 ± 2.26%) and a low average temperature (22.47 ± 1.16 °C). These factors could be the reason for the enhancement of the total carotenoid production during the winter, especially the β-carotene content in T. rigidula. As mentioned above, winter is characterized by low light (Chiang Dao Wildlife Research Station,2018; Wildlife Conservation Office,2015). Despite that, even low light may cause photoinhibition at low temperatures, and high accumulation of carotenoids may occur as a defense mechanism, which has been reported to induce carotenogenesis and enhanced β-carotene production (Mendoza et al.,1996). Ben-Amotz (1996) reported a fourfold increase in the 9-cis/all-trans β-carotene in D. bardawil Ben-Amotz & Avron when the culture temperature was lowered from 30 °C to 10 °C. Furthermore, it was reported the cell membrane integrity is disrupted at low temperatures, thereby making the algae more susceptible to photooxidation (Nishida & Murata,1996). Hence, a high amount of carotenoid accumulates in some microalgae as a protective measure to overcome photoinhibition and oxidative stress (Jahnke,1999; Pisal & Lele,2005).
The low amount of carotenoid content in samples of T. rigidula collected during the rainy and summer seasons could be related to the large amount of rainfall in those seasons, which is typical for the study area. The northern region of Thailand is known as a tropical savanna, which receives a great deal of rainfall during the months of June, July, August, and September; a total rainfall of 1,200.43 ± 15.01 mm was recorded (Chiang Dao Wildlife Research Station,2018; Wildlife Conservation Office,2015). The rainfall, high humidity, and frequent cloud cover may cause the low carotenoid production. Low carotenoid content in various species of Trentepohlia in the mountain of the state of Meghalaya in Northeast India during the rainy season could be owing to the high temperatures and many cloudy days, which generate unfavorable conditions for the growth of Trentepohlia species (Kharkongor & Ramanujam,2015; Mukherjee et al.,2010).
Conclusions
The study of the subaerial green algae genus Trentepohlia in the Chiang Dao Wildlife Sanctuary, Chiang Mai Province, at different sites, altitudes, and substrata showed that the sanctuary had an average relative humidity of 55.97% to 83.93%, an average temperature of 24.23 to 28.27 °C, and an average light intensity of 33.63 to 233.07 µmol photon m−2 s−1. Thirteen species of Trentepohlia were found. The dominant species were T. chapmanii, T. rigidula, Trentepohlia sp. 1, T. effusa, T. abietina, and T. umbrina. The sampling points were separated into four groups according to the similarity of algal species composition found at each point. The diversity of the algae was clearly related to the altitude at which the algae were living, and the associated conditions of relative humidity, temperature, and light intensity. The dominant species, T. rigidula, had a higher total carotenoid content than chlorophyll a and b content. The total carotenoid content, especially of β-carotene, was highest in winter.
Handling Editor
Beata Zagórska-Marek; University of Wrocław, Poland; https://orcid.org/0000-0001-6385-858X