. Introduction
Corticioid fungi is an artificial assemblage of the species from the phylum Basidiomycota and class Agaricomycetes, characterized by effused or effused-reflexed basidiomata, one-celled basidia, and hymenophore of various configurations, from smooth to raduloid and reticulately folded, but excluding poroid and lamellate types. Many of these fungi have flat, thin fruitbodies that develop on dead wood in all seasons, excluding frost periods. They are considered by mycologists as a separate group, accepted historically on the basis of similar macromorphology of basidiomata, ecology, and methodical approaches for the study of species. We accept a brief definition of corticioid fungi (Yurchenko, 2020) as non-poroid resupinate Aphyllophorales, following Jülich and Stalpers (1980), and including one genus of Tremellomycetes, Syzygospora. The latter has a peculiar morphology of basidia, resembling true homobasidia, and the ability of some species to develop very small film-like basidiomata. We also include Dentipratulum, a genus with hydnoid hymenophore, to corticioid fungi, because its fructifications have scarce subiculum (see Holec & Zehnálek, 2021).
The species diversity of corticioid fungi in Poland is relatively well studied. The monograph by Wojewoda (2003) is the most recent catalog of all the larger basidiomycetes, found in Poland. It includes 293 species of corticioid fungi, as defined above. However, new species are recorded almost every year in the country.
Białowieża Primeval Forest is one of the largest and best-preserved non-montane deciduous forest massifs in Europe (Bobiec, 2002). It is an area of particular interest for mycologists (Ruszkiewicz-Michalska et al., 2021) due to its old-aged ecosystems, scarcely modified by human activity, that include both nemoral (associated with, e.g., Quercus robur) and boreal (associated with Picea abies and Pinus sylvestris) elements of biota. Larger basidiomycetes have been studied here since 1826, but a monographic treatment of corticioid fungi has not been realized yet (see Kujawa et al., 2018).
In the present paper, we give the characteristics of some new species, thereby adding to the list of Polish fungi.
. Material and Methods
The new species for Polish mycobiota were identified after microscopic examination of 540 specimens of corticioid fungi, stored in the herbarium of the Institute of Forest Sciences, Białystok University of Technology (BLS, Hajnówka). These specimens were collected by Marek Wołkowycki from the northeast part of Poland, in the period 1993–2022; most collections belong to the years 2018–2020. About 95% of the specimens examined were collected in Białowieża Primeval Forest (southeast part of Podlaskie voivodeship), 1% in Knyszyn Primeval Forest (central-east part of Podlaskie voivodeship), and 4% in Piska Primeval Forest (southeast part of Warmińsko-Mazurskie voivodeship).
Macro- and micromorphology was studied on dry basidiomata. The pictures of fresh basidiomata of some species taken soon after their collection were also used to document macromorphology. For microscopic slides, vertical hand sections of the basidiomata were rehydrated in 3% aqueous potassium hydroxide (abbreviated as KOH in the text). Incrustations on hyphae and hymenial elements and the amyloid reaction of basidiospores were studied in Melzer’s reagent, wherever necessary. Microscopic measurements were done on Nikon Eclipse Ni-U light microscope (Nikon Corp., Japan), mostly under ×1000 magnification, by NIS-Elements Br imaging software (Nikon Corp.). Spore quotient (Q) was determined as the length/width ratio for individual spores.
Scanning electron images (SEM) of selected microstructures of the fungi were obtained on Phenom G2 pro desktop microscope (Labmate, UK). For these images, pieces of fruitbodies were taken from the herbarium, glued to metallic stands using double-sided adhesive film, and coated with a 3.1–3.2 nm layer of gold in a Leica EN ACE200 vacuum coater (Leica Microsystems, Germany).
To confirm that a species was not published for the country before, we used the checklist of Wojewoda (2003) and a checklist in the resource grzyby.pl (Snowarski, 2022). Some additional data on species distribution were taken from the Global Biodiversity Information Facility (https://www.gbif.org).
Nomenclature of the species mostly follows MycoBank (https://www.mycobank.org); systematic position in the hierarchy above genera follows Dictionary of the Fungi system (http://speciesfungorum.org/names/fundic.asp), except Odonticium septocystidiatum. The nomenclature of forest communities follows Matuszkiewicz et al. (2012).
. Results
The study revealed that eight species of fungi identified by us had not been published in articles or monographs for Poland earlier. Besides, we found that one species, Leptosporomyces fuscostratus, required clarification of its status in the Polish biota. The data about these species are given below.
Acanthobasidium norvegicum (J. Erikss. & Ryvarden) Boidin, Lanq., Cand., Gilles & Hugueney (Stereaceae, Russulales)
Syn. Aleurodiscus norvegicus J. Erikss. & Ryvarden
Figure 1
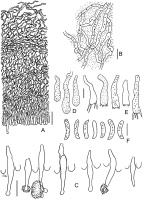
Outer view of basidiomata in dry state: (A) Acanthobasidium norvegicum (BLS M-3535); (B) Amylocorticium laceratum (BLS M-4372); (C) Hyphoderma transiens (BLS M-3260); (D) Leptosporomyces fuscostratus (BLS M-4778); Odonticium septocystidiatum – (E) BLS M-0596, (F) BLS M-0626. Scale bars = 5 mm for A–D, F; 1 mm for E.

Figure 2
SEM images. (A) Acanthobasidium norvegicum BLS M-3535, basidiospore on hymenial surface; (B) Odonticium septocystidiatum BLS M-0626, incrustations on projecting part of cystidia; (C, D) Steccherinum albidum BLS M-1047, pseudocystidia; (E, F) Tubulicrinis calothrix BLS M-0498, apices of cystidia. Scale bars = 10 µm.
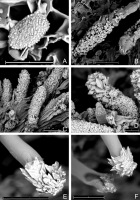
Figure 3
Acanthobasidium norvegicum BLS M-3535: (A) vertical section through basidioma; (B) subicular hyphae; (C) fragment of hymenium; (D) cystidia; (E) acanthophyses; (F) basidia; (G) basidiospores in KOH; (H) basidiospores in Melzer’s reagent. Scale bars: for A, C–H = 10 µm; for B = 5 µm.
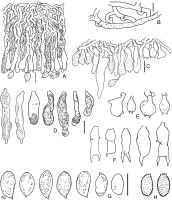
The species is distinguished by the following main features: poorly developed subiculum (10–25 µm thick); numerous subcylindrical or fusoid cystidia, having pale brownish-yellow granular or resinous contents in water and KOH; the presence of acanthophyses; (1)2-sterigmate basidia; large (9–12 µm long) amyloid basidiospores, covered by warts, easily observable in Melzer’s reagent. Some basidia have scarce, short lateral protuberances in their lower half. Occasional basidia have a transverse secondary septum. The acanthophyses of this fungus resemble basidia in shape. Our specimen exhibited mostly 2 apical protuberances on acanthophyses, whereas in pictures published by other authors (Larsson & Ryvarden, 2021; Martini, 2016), the number of protuberances reached 4–7. Spores of this fungus look almost smooth in KOH.
This species is distributed in Western Europe, from Norway and Sweden to Portugal (Bernicchia & Gorjón, 2010; Eriksson & Ryvarden, 1973). Our locality is the easternmost known for the species.
The usual substrata for this species are dead Calluna stems and twigs (Eriksson & Ryvarden, 1973). The fungus was reported on Rubus in France (Wu et al., 2001).
In molecular phylogeny studies (Tian et al., 2018; Wu et al., 2001), it was proposed to belong Aleurodiscus norvegicus to the derivative genus Acanthobasidium together with A. phragmitis Boidin, Lanq., Cand., Gilles & Hugueney, and A. weirii (Burt) L.D. Dai & S.H. He. The generic name indicates that some basidia in A. norvegicus have lateral protuberances, and supposedly acanthophyses are immature basidia (Eriksson & Ryvarden, 1973; Martini, 2016).
Specimen examined: Białowieża Primeval Forest, near Topiło village, compart. No. 574Ch, Vaccinio uliginosi-Pinetum, on dead corticated twigs of Vaccinium uliginosum, 1–5 mm diam., coll. M. Wołkowycki, 2 XI 2019 (BLS M-3535).
Amylocorticium laceratum (Litsch.) Hjortstam & Ryvarden (Amylocorticiaceae, Amylocorticiales)
Figure 4
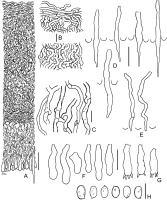
Amylocorticium laceratum BLS M-4372: (A) vertical section through basidioma; (B) subicular hyphae; (C) fragment of hymenium; (D) basidia; (E) basidiospores. Scale bars: for A, C–E = 10 µm; for B = 5 µm.
Syn. Athelopsis lacerata (Litsch.) J. Erikss. & Ryvarden
The species is distinguished by white, pellicular basidiomata, comparatively narrow hyphae [1.3–2.5(–3.5) µm], substipitate-clavate basidia, and allantoid, but relatively broad spores (Q = 2.7–3.3) with well-pronounced apiculus and amyloid reaction. Basidia usually have maximal width near their middle part; sterigmata of basidia are short and wide throughout most of their development period. Spores in our specimen turn bluish with a pale yellow hue in Melzer’s reagent. An additional notable feature is the minutely guttulate contents of hyphae in KOH. Our specimen showed encrusted subhymenium, while this feature was not noted for the species earlier (Eriksson & Ryvarden, 1973; Zmitrovich, 2008). Eriksson and Ryvarden (1973) indicated non-granular protoplasm in the basidioles to be a diagnostic character, but in our specimen, hymenial elements are minutely guttulate or granular in water and KOH. Spores in our specimen (6–8 × 2.7–3.3 µm) are somewhat larger than in descriptions of A. laceratum (6–7 × 2.5 µm; Bernicchia & Gorjón, 2010; Larsson & Ryvarden, 2021).
The species has Eurasian distribution from Norway and Sweden to Spain and Turkey (Bernicchia & Gorjón, 2010; Eriksson & Ryvarden, 1973), as well as in the distant parts of the range in North Urals (Kotiranta & Penzina, 1998) and China (Dai, 2011). Its occurrence is rare in all parts of its natural range (Hjortstam, 1980). The fungus called Athelopsis lacerata was reported by Gates (2009) from Tasmania, but the identity of the Tasmanian material with this taxon is difficult because of the large geographical disjunction.
The fungus grows on decayed coniferous wood (Eriksson & Ryvarden, 1973), especially strongly decayed wood of Pinus, and has also been recorded on Sarothamnus (Larsson & Ryvarden, 2021).
A number of authors put this species in the genus Amylocorticium because of the amyloid spore wall (Hjortstam, 1980; Hjortstam & Ryvarden, 1979; Larsson & Ryvarden, 2021). Molecular data (Binder et al., 2010) confirmed its phylogenetic position in the order Amylocorticiales. However, its generic position is not yet clear; DNA sequences show its relation to Amyloxenasma, whereas basidia and spore shape have similarities with Melzericium.
Specimen examined: Białowieża Primeval Forest, near Topiło village, compart. No. 601B, Salicetum pentandro-cinereae, on dead, mostly corticated branches of Salix cinerea, 7–13 mm diam., and on old thalli of Parmelia sulcata, coll. M. Wołkowycki 18 IX 2020 (BLS M-4372).
Hyphoderma transiens (Bres.) Parmasto (Hyphodermataceae, Polyporales)
The species is distinguished by its odontoid hymenial surface, usually with scattered aculei, and the presence of various tinges of ochraceous, rich crystalline deposits in the subhymenium, scarce subcylindrical enclosed leptocystidia, and middle-sized [(7.5–)8.5–10.5 µm long], cylindrical basidiospores. Micromorphology of this fungus was illustrated earlier by Yurchenko and Kotiranta (2011).
The species has a large natural range, situated predominantly in warm-temperate areas. In Europe, the range extends from Britain, Sweden, and Estonia to Portugal, Italy, Croatia, and Ukraine (Bernicchia & Gorjón, 2010). It was also recorded in Madeira (Telleria et al., 2008), the Azores (Telleria et al., 2009), and the Canary Islands (Beltrán-Tejera et al., 2015). In Asia, it was reported from Russian Caucasus, Georgia, Azerbaijan, northeast Turkey (Ghobad-Nejhad et al., 2009), Iran (Ghobad-Nejhad & Hallenberg, 2012), India (Sanyal et al., 2017), Middle Urals (Shiryaev et al., 2010), China (Dai, 2011), and Japan (Maekawa, 2021). There are records of the material, named H. transiens, from Brazil (Chikowski et al., 2020; Hjortstam & Bononi, 1987), and named Hyphoderma aff. transiens, from Cameroon (Spirin & Ryvarden, 2020). There are two records of this species in GBIF, belonging to Poland, based on specimens in GB herbarium: GB-80037 (collected in 1962) and GB-80036 (collected in 1973).
The fungus grows saprobically mostly on hardwood (Fagus, Quercus), and has also been recorded on Tilia, Cornus (Bernicchia & Gorjón, 2010; Volobuev & Arzhenenko, 2018), and Ulmus (Shiryaev et al., 2010). Our specimens of this fungus indicate a distinct preference to the dead wood of Tilia cordata in Tilio-Carpinetum forest association in the study area.
Specimens examined: Białowieża Primeval Forest, near Hajnówka, compart. No. 386C, Tilio-Carpinetum, on fallen wood of Tilia cordata, coll. M. Wołkowycki, 29 IX 2018 (BLS M-0381); near Czerlonka village, compart. No. 442D, Tilio-Carpinetum, on fallen wood of T. cordata, coll. M. Wołkowycki, 1 VII 2020 (BLS M-3260); near Czerlonka village, compart. No. 418A, Tilio-Carpinetum, on fallen wood of T. cordata, coll. M. Wołkowycki, 10 IX 2020 (BLS M-4304).
Leptosporomyces fuscostratus (Burt) Hjortstam (Atheliaceae, Atheliales)
Figure 5
Leptosporomyces fuscostratus BLS M-4778: (A) vertical section through basidioma; (B) basal hyphae; (C) subicular hyphae; (D) basidioles; (E) basidia; (F) basidiospores. Scale bars: for A = 20 µm; for B–F = 5 µm.
The species is distinguished by the following main features: distinctly pellicular, wide basidioma with dark cream hymenial surface, quickly turning brown-black from KOH; well-developed subiculum, consisting of brown hyphae near substratum; encrusted hyphae in middle subiculum; small-sized basidia [10–12(–13.5) × 3.2–4(–4.5) µm]; lack of cystidial elements; small spores [(3–)3.2–3.7(–4.5) × 2–2.8 µm]. The hyphae of the middle subiculum are pale brown and turn into colorless subbasidial ones. The walls of subicular hyphae can be described as nearly thickened. The wall of many spores looks slightly thickened at ×1000 magnification.
The species has a broad distributional range in the northern hemisphere. In Europe, it extends from Ireland, Norway, and Finland to Spain, Italy, and Croatia (Bernicchia & Gorjón, 2010). In Asia, the localities are scattered from the Caucasus region and Middle Urals to Kamchatka and Primorye (Kotiranta et al., 2016; Shiryaev et al., 2010; Zmitrovich, 2008), China (Dai, 2011), and Japan (Maekawa, 2021). It is known to be found in Canada and USA, including Florida (Ginns & Lefebvre, 1993). There was a record of this species for Poland in GBIF, based on a specimen in the herbarium of Gothenburg University (GB), collected in 1963 (GB-107190).
The fungus grows predominantly on decayed gymnosperm wood: Picea, Pinus, Larix, Abies, Pseudotsuga, Thuja (Bernicchia & Gorjón, 2010; Ginns & Lefebvre, 1993; Kotiranta et al., 2016; Shiryaev et al., 2010; Zmitrovich, 2008). In North America, it was also recorded on Acer and Populus, and considered as a supposedly psychrophilic species (Ginns & Lefebvre, 1993).
The fungus under the name L. fuscostratus was published for Poland only once by Karasiński et al. (2015) from Kampinos and Białowieża national parks. In this source, Confertobasidium olivaceoalbum (Bourdot & Galzin) Jülich is noted as a synonym of L. fuscostratus. However, after the publication by Ginns and Lefebvre (1993), the current name accepted for C. olivaceoalbum is Scytinostromella olivaceoalba (Bourdot & Galzin) Ginns & M.N.L. Lefebvre. The latter is a fungus, having rare skeletal hyphae and fusiform gloeocystidia in the hymenium (Bernicchia & Gorjón, 2010). Both types of elements were not observed in our specimen, and hence we address it as L. fuscostratus, and confirm this species for Polish mycobiota.
Specimen examined: Białowieża Primeval Forest, near Orzeszkowo village, compart. No. 435A, Tilio-Carpinetum, on naked fallen wood of Pinus sylvestris, coll. M. Wołkowycki 2 I 2021 (BLS M-4778).
Odonticium septocystidiatum (Burt) Zmitr. & Spirin (Irpicaceae, Polyporales)
Syn.: Candelabrochaete septocystidia (Burt) Burds.; Phanerochaete septocystidia (Burt) J. Erikss. & Ryvarden
The species is identifiable by its brown ochraceous hymenial surface in mature basidiomata, large (60–145 µm long), semi-immersed cystidia, having 4–11 simple septa, and thus consisting of short cells, absence of clamps at all septa, and small-sized, (5.5–)6–7(–8) × 1.8–2 µm, allantoid basidiospores. Granules of resinous matter are abundant on hyphae and cystidia; this matter is yellow in water but turns brown or orange-brown in KOH. The fungus can develop large basidiomata, 10–20 cm long and more. The micromorphology pictures for this species were published by Eriksson et al. (1978), Martini (2017), Volobuev and Arzhenenko (2018).
The natural range of the species includes mostly warm-temperate areas. It is known to be found in Europe (from Norway and Finland to Spain and Ukraine; Bernicchia & Gorjón, 2010), and the Canary Islands (Beltrán-Tejera et al., 2013). In Asia it was documented in the Caucasus region (Bernicchia & Gorjón, 2010), Iran (Ghobad-Nejhad & Hallenberg, 2012; Ghobad-Nejhad et al., 2009), Middle Urals (Shiryaev et al., 2010), and Kyrgyzstan (under the name Odonticium raitviirii Parmasto; Eriksson et al., 1978). In North America, it was reported from seven states of the USA situated in the north, east, and northeast of the country (Ginns & Lefebvre, 1993). The type locality of the species is in Jamaica (Parmasto et al., 2009). Moreover, there are records from Brazil (Hjortstam & Ryvarden, 2007) and Australia (Bougher & Barrett, 2020).
The fungus grows on decayed wood of angiosperms (Eriksson et al., 1978), including Populus tremula (Volobuev & Arzhenenko, 2018), Tilia and Ulmus (Shiryaev et al., 2010), on the wood and bark of Acer, Betula, Liriodendron, seldom on gymnosperms – Pinus (Ginns & Lefebvre, 1993).
Owing to having clampless hyphae, this species was added to the genus Phanerochaete; because of the presence of septocystidia, it was classified in the genera Candelabrochaete and Odonticium. However, molecular phylogeny studies suggested the natural position of the species in one clade with Ceriporia and Leptoporus (Justo et al., 2017; Li et al., 2022). Consequently, the species seems to belong to the Irpicaceae family.
Specimens examined: Białowieża Primeval Forest, near Hajnówka, compart. No. 384D, Fraxino-Alnetum, on fallen, partly corticated Salix cinerea wood, coll. M. Wołkowycki, 31 X 2018 (BLS M-0596); near Czerlonka village, compart. No. 443Aa, Tilio-Carpinetum, on naked fallen wood of Populus tremula, coll. M. Wołkowycki, 31 X 2018 (BLS M-0626); near Czerlonka village, compart. No. 390A, Ficario-Ulmetum minoris, on dead wood of P. tremula, coll. M. Wołkowycki, 30 IX 2020 (BLS M-4456).
Phlebia cretacea (Romell ex Bourdot & Galzin) J. Erikss. & Hjortstam (Meruliaceae, Polyporales)
Syn.: Cabalodontia cretacea (Romell ex Bourdot & Galzin) Piątek
Figure 6
Outer view of basidiomata: (A) Phlebia cretacea, fresh state (BLS M-3745); Phlebia subulata (BLS M-4040) – (B) fresh state and (C) dry state; (D, E) Steccherinum albidum, dry state (BLS M-1047); (F) Tubulicrinis calothrix, dry state (BLS M-0498). Scale bars = 5 mm for A–D, F; 1 mm for E.

Figure 7
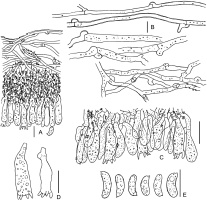
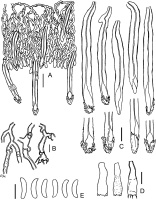
Phlebia cretacea BLS M-3745: (A) vertical section through basidioma; (B) subicular hyphae; (C) cystidia; (D) basidioles; (E) basidia; (F) basidiospores. Scale bars: for A = 20 µm; for B, D–F = 5 µm; for C = 10 µm.
The species is distinguished by the presence of small (21–28 × 3.5–4 µm), capitate cystidia, and narrow, allantoid basidiospores (Q = 4.2–5.4). The capitulum of some cystidia bears a cap of amorphous, colorless matter, easily dissolvable in KOH. Subicular hyphae in our specimen are associated with abundant coccoid algae.
The species’ natural range includes Europe (Norway, Sweden, Finland, Estonia, France, Belgium; Bernicchia & Gorjón, 2010) and North America – Canada, and USA, where it is rare (Ginns & Lefebvre, 1993). An isolated locality in the southern pre-Urals was also reported to have this species (Safonov, 2015). There are four records of this species for Poland in GBIF, based on specimens in GB herbarium, collected in 1973: GB-115560, 115561, 115562, 115563.
The fungus grows on decaying wood of gymnosperms, e.g., decorticated wood of Pinus and Picea (Eriksson et al., 1981) and on Thuja (Ginns & Lefebvre, 1993). It has a tendency to inhabit semi-open habitats (Eriksson et al., 1981).
Specimen examined: Białowieża Primeval Forest, near Czerlonka village, compart. No. 489D, Tilio-Carpinetum, on naked, fallen wood of Pinus sylvestris, coll. M. Wołkowycki, 15 VII 2020 (BLS M-3745).
Phlebia subulata J. Erikss. & Hjortstam
Figure 8
Phlebia subulata BLS M-4040: (A) vertical section through basidioma; (B) hyphae in subicular texture; (C) separate subicular hyphae; (D) cystidia; (E) hyphidia; (F) basidioles; (G) basidia; (H) basidiospores. Scale bars: for A = 20 µm; for B, C, H = 5 µm; for D–G = 10 µm.
The species is distinguished by basidioma of hard corneous consistency in the dry state, densely arranged, narrow [1–2(–3) µm wide] subicular hyphae, presence of scarce subulate leptocystidia, and small-sized [3.7–4(–4.3) × 2.4–2.6(–3.2) µm], ellipsoid basidiospores (Q = 1.5–1.7). Subiculum in our specimen is associated with abundant coccoid algae.
The natural range of this fungus includes Europe (from Norway and Finland to Spain, Serbia, and Ukraine; Bernicchia & Gorjón, 2010), and Middle Siberia (Kotiranta & Shiryaev, 2015). This species has been reported from the Belarusian part of Białowieża Primeval Forest (Yurchenko, 2020). There is a record of the species from Poland in GBIF, based on a specimen from GB herbarium GB-118046, collected in 1973.
The fungus grows on fallen wood of gymnosperms (Picea, seldom Pinus), and is associated with primeval coniferous forests with Hylocomium and Vaccinium (Eriksson et al., 1981). Our specimen was found in a broadleaved forest with an admixture of Picea.
Specimen examined: Białowieża Primeval Forest, between Hajnówka and Topiło village, compart. No. 463A, Tilio-Carpinetum, on fallen, naked wood of Picea abies, coll. M. Wołkowycki, 31 VIII 2020 (BLS M-4040).
Steccherinum albidum Legon & P. Roberts (Steccherinaceae, Polyporales)
The species is distinguished by effused basidioma, whitish hymenial surface with pale ochraceous tinge, comparatively long (0.7–1.7 mm), fairly slender (0.09–0.16 mm diam.), densely arranged (8–11/mm) hymenophoral aculei, and minute [(2.5–)3–3.3 × (1.2–)1.3–1.5 µm], suballantoid basidiospores. The species’ protologue was based on a single specimen (Legon & Roberts, 2002). According to the original diagnosis, the fungus has effused-reflexed basidioma, less dense (5–6/mm), but longer (2–3 mm) aculei, and slightly larger spores [3–3.5(–4) × 1.5 µm], than in our material. Besides, skeletal elements in our specimen were observed only in basal parts of pseudocystidia, and the sterile margin is narrow (about 0.5 mm) or absent. Suballantoid spores are an unusual feature for the genus Steccherinum in general. Our specimen can be named more correctly as S. albidum s.l. until richer material will be collected for this little-known taxon.
The distributional range of the species includes the type locality in Britain, on dead wood of Fagus (Legon & Roberts, 2002), and two localities in Switzerland (GBIF data, occurrences SWISSFUNGI-CH-633787 and -744524). Moreover, a specimen published under the name Steccherinum albidum aff. was found in Mexico (Spirin & Ryvarden, 2016); it has larger spores, than in the European material.
Specimen examined: Białowieża Primeval Forest, between Hajnówka, Topiło village, and Czerlonka village, compart. No. 487B, Tilio-Carpinetum, on decaying bark of Quercus robur, coll. M. Wołkowycki, 18 X 2018 (BLS M-1047).
Tubulicrinis calothrix (Pat.) Donk (Hymenochaetaceae, Hymenochaetales)
Figure 9
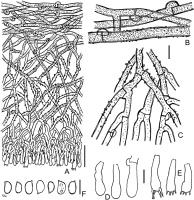
Tubulicrinis calothrix BLS M-0498: (A) vertical section through basidioma; (B) subicular hyphae; (C) cystidia and their apices; (D) basidia; (E) basidiospores. Scale bars: for A = 20 µm; for B, D, E = 5 µm; for C = 10 µm.
The species is distinguished by lyocystidia, which have apically asymmetrically thickened wall and often bear a cap of crystals that are partially dissolvable in KOH. Some amount of cystidia have symmetrical wall thickening; sometimes, this symmetry depends on cystidium projection on the microscopic slide. Thus for reliable species identification, at least 20 cystidia should be studied.
The species has hemicosmopolitan distributional range, with a preference for warm-temperate regions. Type locality of it is in Tunisia, North Africa (Parmasto et al., 2009). In Europe, it is known to be found from Norway and Finland to Portugal, Italy, and Greece (Bernicchia & Gorjón, 2010). Asian part of the range includes Turkey (Bernicchia & Gorjón, 2010), Middle Urals (Shiryaev et al., 2010), Middle and East Siberia (Kotiranta & Shiryaev, 2015; Kotiranta et al., 2016; Shiryaev & Kotiranta, 2015), Primorye (Viner & Kokaeva, 2017), China (Dai, 2011), and Japan (Maekawa, 2021). In North America, it is known to occur in Canada and USA (Ginns & Lefebvre, 1993). Other regions where the species was found include the Canary Islands (Beltrán-Tejera et al., 2013), Venezuela (Liberta & Navas, 1978), Hawaii (Gilbertson et al., 2001), and Réunion (Hjortstam & Ryvarden, 2007). There were 7 records of this species in GBIF, belonging to Poland, based on specimens in GB herbarium and collected in 1973: GB-100377, 100378, 100379, 100380, 100381, 100382, 100383.
The fungus grows mostly on dead wood of Pinus, Picea, Larix, Abies (Bernicchia & Gorjón, 2010; Kotiranta & Shiryaev, 2015; Kotiranta et al., 2016; Shiryaev & Kotiranta, 2015; Shiryaev et al., 2010); in North America, it is known to grow on Pseudotsuga, Tsuga, Quercus (Ginns & Lefebvre, 1993); in the Canary Islands it occurs on unusual substrata like Euphorbia (Beltrán-Tejera et al., 2013) and Cystus (Beltrán-Tejera et al., 2015). In Poland, the fungus was found on an unusual host, too (Populus tremula).
Specimen examined: Białowieża Primeval Forest, near Czerlonka village, compart. No. 543Ba, Peucedano-Pinetum, on naked, fallen Populus tremula wood, coll. M. Wołkowycki, 6 XI 2018 (BLS M-0498).
. Discussion
The number of species of fungi within a country is not a fixed value, since new species are added almost every year to the country list. This can be attributed to the inclusion of new areas for collection, a thorough examination of habitats, studying more herbarium material, taking into account new taxonomic publications, the growth of taxonomic experience of mycologists, and supposedly also the expansion of the natural ranges of the species due to changes in the environment. The effectiveness of the process of studying corticioid fungal diversity in Poland can be evaluated from our data. Most of the herbarium material from this study was collected over three years and only from three forested areas in the northeast part of the country. The research added eight species to the biota: Acanthobasidium norvegicum, Amylocorticium laceratum, Hyphoderma transiens, Odonticium septocystidiatum, Phlebia cretacea, Ph. subulata, Steccherinum albidum, and Tubulicrinis calothrix, which constitutes about 3% of the total corticioid fungal diversity known in 2003 (Wojewoda, 2003). The specimens of new species constitute 2.2% of the total number of collections examined. All nine species described in this paper were found in Białowieża Primeval Forest, but they are still not recorded in Knyszyn and Piska Primeval Forests. It should be noted, that all nine species were found outside of the borders of Białowieża National Park, a core of Primeval Forest, which is a specially protected area with strict regulations for collecting fungi. Of the nine species described above, only Phlebia subulata was known from the Belarusian part of Białowieża Primeval Forest.
We have found that the records of five species from Poland (Hyphoderma transiens, Leptosporomyces fuscostratus, Phlebia cretacea, Ph. subulata, Tubulicrinis calothrix) were added earlier in the GBIF database (https://www.gbif.org). All of them were based on specimens from the GB herbarium (University of Götheborg, Sweden). We confirmed these species from the recently collected original material.
From nine species discussed, six (Amylocorticium laceratum, Hyphoderma transiens, Leptosporomyces fuscostratus, Odonticium septocystidiatum, Ph. subulata, Tubulicrinis calothrix) have Eurasian or broader distribution range, and three (Acanthobasidium norvegicum, Phlebia cretacea, Steccherinum albidum) have European or Euro-American distribution. Two species (Hyphoderma transiens, Odonticium septocystidiatum) occur predominantly in warm-temperate regions belonging to nemoral and Mediterranean biomes. Along with identified species, about 14% of the studied specimens still belong to unclear material without specific and sometimes without generic epithet and this suggests the need for further biodiversity studies.


