. Introduction
Chickpea (Cicer arietinum L.) is one of the oldest cultivated protein legumes in the world. It is mainly used for human consumption and is an essential constituent of the Mediterranean diet and basic food in Pakistan and India (Millan et al., 2010). Domesticated in association with other crops such as wheat and barley, Cicer arietinum L. is believed to be a part of the agricultural revolution, and in terms of consumption, ranks second after broad bean (Gupta et al., 2014).
In Morocco, the cultivated chickpea area covers an acreage of 54,000 ha (MAPMDREF, 2020a), with a production of 49,700 T recorded in the 2019/2020 agricultural season (MAPMDREF, 2020b). Most of the agricultural systems of chickpea production in the country suffer from several constraints, mainly biotic and abiotic factors that cause serious damage before and after harvest. The widespread high temperature and drought stress in different regions of chickpea production can affect flowering and pod setting stages which lead to decreases in chickpea yield (Houasli et al., 2020). Previous reports on the chickpea crop have recorded the presence of Ascochyta rabiei, the causal agent of blight disease in Morocco, in all chickpea areas (Grewal, 1984; Singh, 1984). Bencheqroun et al. (2022) pointed out that Didymella rabiei (Kovatsch.) Arx. is the most devastating fungal infection of chickpea crops inflicting considerable yield and quality losses. Moreover, numerous fungal species associated with the chickpea diseases of wilt and root rot have been reported in Morocco, including Fusarium oxysporum f sp. ciceris (El Aoufir, 2001; Elbouazaoui et al., 2018); Fusarium redolens (Jiménez-Fernández et al., 2011); and Rhizoctonia bataticola, R. solani, and Pythium sp. (Elbouazaoui et al., 2018).
Fusariumequiseti has also been reported in other crop species, especially melon (Cucumis melo), soybean (Glycine max), cumin (Cuminum cyminum), cauliflower (Brassica oleracea), winter rapeseed (Brassica napus), tomato plant (Solanum lycopersicum), pepper (Capsicum annuum), and the cabbage Brassica oleracea (Adams et al., 1987; Chen et al., 2014; Gally et al., 1998; Goswami et al., 2008; Li et al., 2014; Ramchandra & Bhatt, 2012). The pathogen is also responsible for the pre- and postharvest decay of zucchini fruits (Cucurbita pepo L.) (Ezrari et al., 2020). It was recently isolated from the seeds of the fragrant wallflower (Matthiola longipetala), showing rot (Ivanović et al., 2020). Khan et al. (2021) noted that F. equiseti was responsible for seedling death in sugar beet.
Isolates of Fusarium and F. solani were recovered from necrotic lesions of chickpea roots in different chickpea growing areas (El Hazzat et al., 2019). Nevertheless, taxonomic confirmation of which species of Fusarium causes this necrosis is lacking because numerous species are important plant pathogens (Austwick, 1982). Additionally, the differentiation of Fusarium species through morphological characters is imprecise; hence, the use of molecular techniques becomes more efficient and accurate for the discrimination of fungal species (Steenkamp et al., 2000).
Therefore, the present study was carried out to identify an isolate of Fusarium sp. collected for the first time in chickpea fields in Morocco from diseased chickpea plants based on morphological characters and molecular and pathological characterization via the fulfillment of Koch’s postulates.
. Materials and Methods
Fungal Material
Fusarium isolates were obtained from necrotic lesions associated with infected stem samples of chickpea plants that were grown in different fields in Souk Tlat in Gharb Province of the Rabat-Sale-Kenitra region, Morocco. One hundred plants were chosen at random from the fields. One stem base sample from each plant was analyzed.
Pieces of diseased tissues were rapidly disinfected with 90% alcohol for 5 min, rinsed three times with sterile distilled water, and dried with sterile filter paper. Samples were then placed onto potato dextrose agar plates (BIOKAR Diagnostics) and incubated at 25 °C for 7 days. The colonies formed were transferred to a potato sucrose agar (PSA) medium containing potato, sucrose, agar-agar, and distilled water. Agar plates were incubated in the same conditions and then observed for species determination. The Fusarium sp. isolate was cultivated in Petri dishes containing PSA medium. The medium was poured into Petri dishes containing 100 mg/L of chloramphenicol at a rate of 30 to 40 mL per dish. Incubation of cultures was performed in the dark at 25 °C for 7 days, followed by macroscopic and microscopic characterization depending on the age of the cultures.
Morphological Characterization
A macroscopic examination of Fusarium sp. was carried out according to the development of the cultures on the PSA medium. Observations focused on colony appearance, mycelium density, the presence of the pinkish color of the colony, as well as growth and spore production. The microscopic characteristics of the Fusarium N3 isolate were determined under an optical microscope to confirm the species identity of this pathogen.
Molecular Analysis and Identification
Molecular identification of the Fusarium N3 isolate was performed after 5 days of culture on PSA medium. DNA extraction was performed according to the method described by Murray and Thompson (1980) and Doyle and Doyle (1987). DNA amplification of the internal transcribed spacer (ITS) rDNA region was performed using polymerase chain reaction (PCR) using universal primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (White et al., 1990). The PCR reaction was carried out in a reaction mixture of 25 µL containing 5 µL of 5× buffer (MyTaq Reaction Buffer, Bioline, London, UK), 1 µL of each primer (10 µM), 0.2 µL of MyTaq DNA polymerase (Bioline, London, UK) (5 U µL−1), 1–2 µL of template DNA (100 ng) and Milli-Q water to complete the volume. A Veriti thermal cycler (Applied Biosystems) was used for the PCR with the following conditions: initial denaturation at 95 °C for 1 min; 35 cycles of denaturation at 95 °C for 15 s, annealing at 52 °C for 20 s, and extension at 72 °C for 15 s; and a final elongation of 72 °C for 3 min. The quality of the PCR products was verified by electrophoresis on 1% agarose gel in the presence of a 100 bp molecular weight marker. Sequencing was performed using an ABI PRISM BigDye Terminator v.3.1 Ready Reaction Cycle Sequencing Kit and primer set ITS1 and ITS4. The sequencing products were run on an ABI PRISM 3130XL Genetic Analyzer (Applied Biosystems) using the POP-7 polymer. The sequence resulting from this study was submitted to GenBank under accession no. MT111122.
The obtained ITS sequence was then compared with the homologous nucleotide sequence in the GenBank database using the Basic Local Alignment Search Tool (BLAST) (http://www.ncbi.nlm.nih.gov/BLAST).
The seeds of a local variety of chickpeas intended for pathogenicity testing were surface sterilized by soaking for 5 min in a 10% NaOCl solution, rinsed in sterile distilled water, and then dried on filter paper. The pathogenicity of the N3 isolate was checked through two inoculation techniques. Technique 1: The surface-disinfected chickpea seeds were inoculated by soaking in water containing a conidial suspension of 106 spores/mL of the Fusarium isolate N3 at room temperature (20 °C) for 1 h. The control seeds were treated with only sterile distilled water. The inoculated and control chickpea seeds were sown in plastic pots (13 cm × 13.5 cm) containing autoclaved sieved Mamora forest soil at the rate of 5 seeds/pot. The Mamora forest soil used is loose, very sandy, and slightly basic pH (7.27), with an organic carbon content of 0.35% to 0.6% (Mouria, 2009). The soil was sieved and sterilized three times at an interval of 24 h at 200 °C for 2 h and then distributed in the plastic pots (13 cm × 13.5 cm) at the rate of 2 kg of soil per pot.
Technique 2: The culture substrate was inoculated by pouring 15 mL of the conidial suspension of the N3 isolate (Fusarium) at a concentration of 106 spores/mL into each pot containing sterile soil. The fungus was allowed to settle for 48 h in the growing medium. The previously disinfected chickpea seeds were sown into these pots at the rate of 5 seeds per pot. Three replicates were prepared for each of the treatments (each pot was a replicate). Two lots of chickpea cultivation pots (control and inoculated) were brought back to the greenhouse to promote seed germination, plant growth, and symptom development monitoring.
After 4 weeks, plant emergence and plant survival were determined in pots containing inoculated seeds. In the pots with inoculated culture substrate according to the second inoculation technique, plant emergence and disease symptoms on chickpea plants were noted after 8 weeks, followed by an assessment of disease severity by calculating the leaf damage index according to the scale established by Douira and Lahlou (1989).
| Notes | Appearance of leaves |
|---|---|
| 0 | Healthy appearance |
| 1 | Cotyledonary leaf: wilting or yellowing |
| 2 | Cotyledonary leaf: fall |
| 3 | True leaf: wilting or yellowing |
| 4 | True leaf: necrosis |
| 5 | True leaf: fall |
The scores related to the number of leaves constitute the foliar alteration index, calculated according to the formula below (Douira & Lahlou, 1989):
FAI = [Σ (i × Xi)] / 6 × NtF
FAI: Foliar alteration index.
i: Leaf appearance notes 0–5.
Xi: Number of leaves with note i.
NtF: Total number of leaves.
An average index was then calculated for each batch of plants.
At the end of the trial, the pots were brought back to the laboratory to re-isolate the pathogen from the different parts of the plants (roots, crown, stems, and leaf petioles) obtained either from the inoculated seeds (technique 1) or inoculated growing substrate (technique 2). The different parts were separated and disinfected with 95% alcohol for 2 min. Samples were then rinsed several times with sterile distilled water, dried quickly on sterile filter paper, transferred to a PSA medium, and incubated in the dark at 25 °C. The microscopic observation was performed after 1 week.
The re-isolation percentage (RP%) was calculated using the following formula: RP = (Ns PX/NT) × 100, where Ns PX is the number of segments containing the fungal species X and Nt is the total number of segments used for re-isolation.
After 8 weeks, the lengths of the aerial and root parts of the chickpea plant were measured with a double decimeter, and the number of leaves and pods of each plant was counted. The fresh weights of the aerial and root parts were recorded using a precision balance and the dry weight of these parts was recorded after being dried in the oven at 70 °C for 48 h.
. Results
The isolate N3 of Fusarium equiseti was obtained from symptomatic samples among a complex of Fusarium species, including F. solani and F. oxysporum. The percentage of isolation was 3% in the case of F. equiseti.
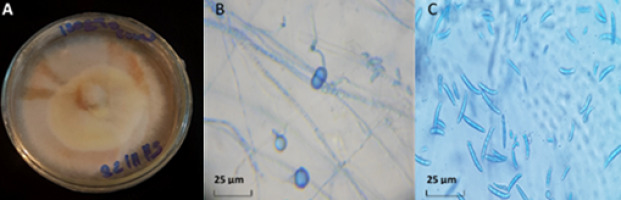
On the PSA medium, isolate N3 developed a colony with abundant aerial mycelium which was fluffy and beige-white colored (Figure 1). Under a microscope, the mycelial filaments were septate. Chlamydospores were present (7 to 13 µm in diameter, spherical, globular, most often intercalary, solitary, or in pairs, and frequently as short chains). Macroconidia were numerous, slightly curved, usually with 5 to 6 septa and 31 to 45 µm long. The description was identical to that of F. equiseti (Corda) Saccardo, reported by Leslie and Summerell (2006) and Rafique et al. (2019). The identity of the isolate N3 was evaluated and confirmed. After identification, the isolate N3 was registered in the national database under voucher ID: RAB111030 and submitted to GenBank as accession no. MT111122. This sequence was 99.33% identical to Fusarium equiseti.
Figure 1
Characteristic of Fusarium equiseti colony (A); chlamydospores (B); macroconidia and microconidia (C). Magnification 400×; mounting medium, lactophenol cotton blue.
The results of the pathogenicity tests using two inoculation methods demonstrated the pathogenic capacity of the isolate N3 of F. equiseti towards a local variety of chickpeas. Few plants emerged from seeds inoculated with the isolate N3. The majority of inoculated seeds turned rotten (Figure 2) compared to un-inoculated seeds, which grew normally under greenhouse conditions.
Figure 2
Symptoms of stunting in chickpea plants (a) derived from seeds inoculated with Fusarium equiseti isolate (F.e) and control seedlings from non-inoculated chickpea seeds (T); (b) symptoms of rot in inoculated chickpea seeds; (c) brownish discoloration of the crown in chickpea plants grown in F. equiseti-infested soil (F.e); (d) chickpea plants grown in un-infested soil (T).

Furthermore, chickpea plants obtained from seeds inoculated with F. equiseti showed a disruption in the growth parameters. The length of the plants, the average number of leaves produced as well as the average number of branches formed were much lower in plants from inoculated seeds than those presented by the control plants; these parameters were 1.29 and 29.05 cm, 1.11 and 24.21 leaves, and 0 and 3.50 twigs, respectively. Fusarium equiseti isolate also adversely affected the growth of roots which exhibited a root length of 0.91 cm, and 0.78 and 0.13 g as fresh and dry weight of roots, respectively, in comparison with 27.16 cm, 7 g, and 5 g, respectively, in the control plants.
The symptoms observed in chickpea plants developed on a culture substrate inoculated with F. equiseti according to technique 2 were variable: necrosis of the roots and crown followed by wilting of the plants. After 8 weeks of cultivation, the aggressiveness of infection induced by the F. equiseti isolate, as estimated by calculating the foliar alteration index, was 0.395 in plants grown in the culture substrate inoculated with a conidial suspension of the Fusarium isolate N3.
Regarding growth parameters, chickpea plants grown in the inoculated substrate displayed a length of 19.44 cm, an average of 18.66 leaves, and 2.08 twigs per plant compared to a length of 31.05 cm, 25.66 leaves, and 4.11 twigs per plant in control chickpea plants.
Plants that were grown in the culture substrate inoculated with F. equiseti also presented a lower root length, fresh and dry weight of root or aerial parts, attaining 19.73 cm, 5.33 and 4.12 g, and 5.47 and 4.02 g, respectively, than plants grown in uninoculated soil (28.33 cm, 7.33 and 6.60 g, and 6.66 and 5.40 g, respectively).
The re-isolation performed on the plants grown either from inoculated seeds or on soil infested with F. equiseti confirmed the presence of the fungus in different parts of the plants. Using the first technique, the highest percentage of the pathogen re-isolation was registered in the chickpea root at 70.22%, followed by collar (70%), stem (60%), and leaf petiole (34.33%), whereas percentages recorded in chickpea plants after soil inoculation were 84.77% (collar), 72.77% (root), 64.44% (stem), and 36.11% (petiole). Fusarium equiseti was consistently reisolated from infected seeds and collar tissues, satisfying Koch’s postulates. To our knowledge, this is the first report of Fusarium equiseti causing seed rot and discoloration at the level of the crown and wilting of chickpea plants.
. Discussion
The Fusarium complex responsible for root rot and wilt diseases in chickpeas is diverse (Zemouli-Benfreha et al., 2014). One of the representative candidates of this complex is the F. equiseti which was isolated for the first time in Morocco from the roots of diseased chickpea plants and identified using morphological characters in addition to molecular characterization. In the current study, Koch’s postulates were verified by inoculation of chickpea seeds and culture substrate.
The inoculated seeds expressed weak germination and growing ability with a high degree of rotting and decomposition. Chickpea plants from seeds sown in soil infested with a suspension of F. equiseti spores showed a range of deterioration symptoms such as root and crown necrosis, vascular browning, yellowing leaves, and stunting and wilting of the plants.
The fungus was re-isolated from the roots, crown, stems, and leaf petioles of the inoculated plants through seed dip inoculation or soil infestation with the spore suspension of F. equiseti. The ability of the pathogen to invade the upper levels of plants can be inferred from these results. Colonization of chickpea plant tissues by F. equiseti after inoculation affected their growth, leading to the root and vegetative growth retardation, including the production of leaves and twigs. All these types of symptoms were observed in chickpea plants inoculated with F. solani (El Hazzat et al., 2019) as well as in lentil and cumin (Cuminum cyminum) plants infected with F. equiseti (Rafique et al., 2019; Ramchandra & Bhatt, 2011). Fusarium equiseti is among the fungal species capable of attacking several legume species, in which it can induce damping-off and root rot disease (Rubella et al., 2008). These authors have reported decayed seeds and reddish brown to black lesions on hypocotyl and roots of kidney bean (Phaseolus vulgaris), pea (Pisum sativum), and chickpea (Cicer arietinum) following inoculation with F. equiseti isolates originating from fields of ginseng. Similarly, the seeds that germinated in soil infected with F. equiseti resulted in plants that showed browning at the crown and stem base followed by wilting. Some symptoms on leguminous hosts bore a resemblance to those stated herein. On wild pigeon peas, this fungal species provokes foliar chlorosis, browning and black discoloration of the stem, plant drying, and, ultimately, plant death (Mishra et al., 2021). Fernandez and Jefferson (2004) observed a discoloration in the subcrown stems and roots of cereal species.
This fungal species is capable of infecting seeds, roots, tubers, and fruits of several species of cultivated plants, such as cucurbits (Joffe & Palti, 1967), cotton (Chimbekujwo, 2000), cowpea (Rodrigues & Menezes, 2005), lentils (Chaudhary & Kaur, 2002), sugar beet (Stojsin et al., 2001), potatoes (Rai, 1979; Theron & Holz, 1989), citrus (Sukmawati & Miarsyah, 2017), pine (Ocamb & Juzwik, 1995), and even nursery plants (Bloomberg, 1981).
Fusarium equiseti has also been isolated from cereals such as corn, wheat, and barley (Ballois, 2012). This fungal species is responsible for rotting stems and premature wilting of corn plants (Swamy et al., 2020) and root rot in winter wheat (Booth, 1971). Sometimes, this pathogen is associated with the blight of wheat ears (Gale, 2003; Shaner, 2003; Tekauz et al., 2009; Wing et al., 1993; Xue et al., 2006) and rice panicles. Infection can also occur during grain storage or afterward (Hashem et al., 2010). In Morocco, several Fusarium species are associated with symptoms of root rot in cereals; the most common are F. equiseti, F. culmorum, F. oxysporum, and F. solani (Lyamani, 1988).
Fusarium equiseti, isolated for the first time in Morocco, was isolated from the fungal complex associated with chickpea roots. Pathogenicity tests conducted in the greenhouse showed that this fungal species is endowed with significant pathogenicity towards this host plant, and the symptoms developed were similar to those observed in chickpea plants inoculated with F. solani (El Hazzat et al., 2019). Extending the surveys to other regions of Morocco is important to build a population of F. equiseti isolates and to determine the amplitude of the variation in the pathogenicity of this pathogen via other chickpea varieties grown in Morocco. With time and in the absence of an effective control program, Fusarium equiseti will probably become an important pathogen and take its place among the other known diseases of chickpeas.


