. Introduction
Fungicolous fungi (or mycophilic fungi) represent a very large, diverse, ecological, and trophic group of organisms that are associated with other fungi (Gams et al., 2004; Sun et al., 2019). Interspecific relationships between fungicolous fungi and their hosts can be characterized broadly as neutralistic, mutualistic, or antagonistic/competitive; however, this term applies even when the relationship between fungi is not fully known (Gams et al., 2004). Wherever fungi have been found, fungicolous taxa have also been identified. So far, 1,552 taxa of fungi have been registered across the world, living on the thallus of other fungi and entering into various, often changing, and complicated relationships (Sun et al., 2019). In Poland, in recent years, such a phenomenon concerning the prevalence of fungi on the parasitic fungi of plants has been reported by, among others Adamska and Czerniawska (2010), Adamska et al. (2010), Bartkowska (2007), Czerniawska (2001, 2005), Czerniawska and Adamska (2010), Dolińska et al. (2011), Górzyńska et al. (2018, 2023), Mułenko et al. (2008), Piątek (2003), Płachecka (2005), Ruszkiewicz-Michalska (2010), as well as Sucharzewska et al. (2011, 2012a, 2012b). The major representatives of the fungicolous fungi include mycoparasites or superparasites that enter into strict biotrophic or necrotrophic relationships, leading to host fungus destruction (Gams et al., 2004; Sun et al., 2019). They can significantly affect the dynamics and size of their host populations in different ecosystems, including both natural and anthropogenic ones. In addition to antagonism and competition, the ability of microorganisms to superparasitism is a natural mechanism that plays an important role in the functioning of natural communities and those exposed to strong anthropopressure (Parratt & Laine, 2016). A superparasite organism (hyperparasite) uses another organism, which is a parasite, to grow and develop, entering into various relationships with it in the host-parasite-hyperparasite system (Boosalis, 1964; Jeffries, 1995). This may be of particular importance in the case of plant diseases induced most often by fungi as the etiological factor. Given the integrated method of plant protection in force since 2014, great emphasis is placed on the search for new, effective organisms exhibiting, among others, the above-mentioned capabilities. This issue has been extensively addressed in agricultural sciences, focusing mainly on economically important plants (Sood et al., 2020; Thambugala et al., 2020), whereas ample studies conducted across the world have aimed to develop bio-preparations for the eradication of dangerous plant pathogens (Grzegorczyk et al., 2015; Knudsen & Dandurand, 2014; Pal & Gardener, 2006; Prajapati et al., 2020; Wachowska et al., 2017). Despite so many investigations on natural antagonistic interactions between microorganisms, the phenomenon of hyperparasitism in the urbicenosis is still poorly understood. So far, there have been few such studies on the presence of superparasites on plant pathogens in cities (Czerniawska & Adamska, 2007; Czerniawska et al., 2011; Madej & Antoszczyszyn, 1965; Ruszkiewicz-Michalska, 2010; Sucharzewska et al., 2011, 2012a, 2012b), and they have mainly aimed at observing the emergence of pathogens on plants, their biology and their impact on the health of host plants, with no account taken of the prevalence of antagonistic microorganisms (Adamska, 2012, 2019; Frużyńska-Jóźwiak & Andrzejak, 2007; Mazurkiewicz-Zapałowicz et al., 2012; Piątek, 2003; Werner & Gołębniak, 2010). The city is a dynamic biocenosis with specific and continuously changing conditions, where the introduction of new, sometimes invasive plant species also promotes the presence of parasites, reducing the aesthetic and recreational values of the urban vegetation. Therefore, in addition to monitoring the emergence of new species of parasitic fungi, it seems important to monitor the prevalence of fungicolous fungi, including fungal superparasites occurring on urban vegetation, which in the future could indicate trends in the development of biological methods for plant protection in this environment.
The aim of the present study was to analyze the prevalence of fungicolous fungi, with particular attention paid to the phenomenon of superparasitism, as well as to assess the species diversity of microorganisms colonizing the thallus of fungi infesting plants in the urban environment.
. Material and methods
The research was conducted in 15 cities in north-eastern Poland, in the Warmian-Masurian Province (Figure 1), during the growing seasons of 2018–2019 from June to September (as part of the research task no. 2017/01/X/NZ8/00798). The experimental material included leaves showing signs of infestation by fungi (spots, necrotic spots, rot). The collected plant material was transported to the laboratory and preserved. After drying, fragments of plants were packed in paper envelopes. Fungal parasites of plants and fungicolous fungi were detected and identified using a stereoscopic microscope and a light microscope. Infested parts of plants were observed under a stereoscopic microscope (Olympus SZX 9) to determine the developmental stage of fungi, and then visible etiological signs were removed from the infested areas using a preparation needle. In the case of morphological structures of fungi embedded in the plant tissue, cross-sections were made and microscopic analyses were carried out under the Olympus BX41 light microscope, additionally using the 1000x immersion lens. Structure measurements were performed using Analysis software.
The fungi were identified using the following keys: Borowska (1986), Brandenburger (1985), Braun (1995), Braun et al. (2003), Ellis and Ellis (1985), Majewski (1977, 1979), Mel’nik (2000), Sałata (1985, 2002), Sutton (1980) and Wołczańska (2013). The nomenclature of fungi was adopted after Index Fungorum. The nomenclature of host plants was adopted after Mirek et al. (2002).
. Results
In total, 411 samples were collected for the needs of this study. Parasitic fungi of plants were detected in 296 samples (72%). Throughout the study, fungicolous fungi were found on the thallus of these parasitic fungi; they were identified in 105 samples (which accounts for 35% of all samples tested). 127 species of microscopic parasitic fungi found on the plants have been identified. They belonged to Ascomycota (with Erysiphales as the prevailing order), Basidiomycota (with Puccinales as the prevailing order), and also to Anamorphic Fungi.
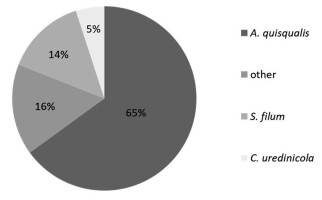
Fungicolous fungi belonged to 12 species: Alternaria alternata, Ampelomyces quisqualis, Aureobasidium pullulans, Cladosporium uredinicola, Cladosporium cladosporioides, Cladosporium herbarum, Clonostachys epichloë, Epiccocum nigrum, Fusarium avenaceum, Sphaerellopsis filum, Stemphylium sarciniforme, and Tripospermum myrti. Fungicolous fungi have been detected on 33 species of fungi parasitic to plants from 43 species (Table 1). Of the micofilic fungi four species were classified as superparasites: Ampelomyces quisqualis, Cladosporium uredinicola, Clonostachys epichloë, and Sphaerellopsis filum. The most common was the hyperparasite of powdery mildew, i.e., A. quisqualis – detected in 68 samples (i.e., in 65% of all analyzed samples). The second most common was the Pucciniales superparasite, Sphaerellopsis filum, detected in 15 samples (14%), followed by Cladosporium uredinicola identified in five samples (5%). The remaining fungicolous fungi occurred in 17 samples (16%); however, they were recorded sporadically in single samples (Figure 2).
Table 1
Fungicolous fungi found on various species of parasitic fungi on host plants during the period of the study.
| No. | Fungicolous fungi | Host parasite [developmental stage] | Host plant |
|---|---|---|---|
| 1 | Alternaria alternata (Fr.) Keissl. | Erysiphe palczewskii (Jacz.) U. Braun & S. Takam. [ma] | Caragana arborescens Lam. |
| *Puccinia malvacearum Bertero ex Mont. [III] | Alcea rosea L. | ||
| *Phyllactinia fraxini (D.C.) Fuss [ma] | Fraxinus excelsior var. monophylla (Dum.Cours.) Gren. & Godr. | ||
| 2. | Ampelomyces quisqualisCes. | Erysiphe alphitoides (Griffon & Maubl.) U. Braun & S. Takam. [mc, c, ya] | Quercus robur L. |
| Erysiphe berberidis DC. [mc, c, ya] | Berberis vulgaris L., B. thunbergii DC., Mahonia aquifolium Pursh | ||
| Erysiphe cichoracearum DC. [mc, c, ya] | Solidago canadensis L. Helianthus tuberosus L. | ||
| *Erysiphe deutziae (Bunkina) U. Braun & S. Takamatsu [mc, c] | Deutzia scabra Thunb. | ||
| Erysiphe flexuosa (Peck) U. Braun & S. Takam. [mc, c, ya, ma] | Aesculus hippocastanum L.; Aesculus × carnea Zeyh | ||
| *Erysiphe hedwigii (Lév) Braun & S. Takam. [mc, c] | Viburnum lantana L. | ||
| Erysiphe hypohylla (Nevod) U. Braun & Cunningt [mc, c, ya] | Q. robur | ||
| E. palczewskii [mc, c, ya] | C. arborescens | ||
| Erysiphe polygoni D.C. [mc, c, ya] | Polygonum aviculare L. | ||
| *Erysiphe syringae-japonicae (U. Braun) U. Braun & S. Takam. [mc, c, ya] | Syringa vulgaris L. | ||
| Erysiphe trifolii Grev. [mc, c, ya] | Lupinus polyphyllus L. | ||
| *Erysiphe urticae [mc, c, ya] | Urtica dioica L. | ||
| Golovinomyces sordidus (L. Junell) V.P. Heluta [mc, c, ya] | Plantago major L. | ||
| Microsphaera vanbruntiana var. sambuci racemosae U. Braun [mc, c, ya, ma] | Sambucus racemosa L. | ||
| Phyllactinia guttata (Wallr. Ex Fr.) Lév. [mc, ya] | Betula pendula Roth | ||
| Phyllactinia fraxini (DC.) Fuss [mc, ya] | F. excelsior ‘Monophylla’ | ||
| Podosphaera fusca (Fr.) U. Braun & Shishkoff [mc, c, ya] | Taraxacum officinale F.H. Wigg. | ||
| Sawadaea tulasnei (Fuckel) Homma [mc, ya] | Acer platanoides L. | ||
| *Uncinula adunca (Wallr) Lév. [mc, c, ya] | Salix cinerea L. | ||
| 3. | Aureobasidium pullulans (De Bary) G. Arnaud | Erysiphe alphitoides [mc, ya, ma] | Q. robur |
| Erysiphe palczewskii [mc, ya, ma] | C. arborescens | ||
| 4. | Cladosporium uredinicola Link | Cronartium flaccidum (Alb. & Schwein) G. Winter [III] | Asclepias syriaca L. |
| Cronartium ribicola H.A. Dietr [III] | Ribes sp. | ||
| *Puccinia arenariae (Schumach.) G. Winter [III] | Dianthus sp. | ||
| *Puccinia glechomatis DC. [III] | Glechoma hederacea L. | ||
| *Puccinia malvacearum Bertero ex Mont. [III] | Alcea rosea L. | ||
| 5. | Cladosporium cladosporioides (Fresen.) G.A. de Vries | *Erysiphe berberidis [mc, c, ya, ma] | Berberis vulgaris |
| *Phyllactinia guttata [ma] | Betula pendula | ||
| *Phyllalctinia fraxini [ma] | Fraxinus excelsior | ||
| *Sawadaea bicornis (Wallr.) Homma [mc, ma] | Acer campestre L. | ||
| 6. | Cladosporium herbarum (Pers.) Link | Erysiphe palczewskii [ma] | C. arborescens |
| 7. | Clonostachys epichloë Schroers | Epichloë typhina (Pers.) Brockm [stroma] | Puccinellia distans L. |
| 8. | Epicoccumnigrum Link | *Puccinia malvacearum Bertero ex Mont. [III] | Alcea rosea L. |
| 9. | Fusarium avenaceum | *Puccinia malvacearum [III] | A. rosea |
| 10. | Sphaerellopsis filum (Biv.) B. Sutton | Coleosporium tussilaginis (Pers.) Lév. [II] | Sonchus arvensis L. |
| Cumminsiella mirabilissima (Peck) Nannf. [II] | M. aquifolium | ||
| Cronartium ribicola J.C. Fisch. [III] | Ribes sp. | ||
| *Melampsora larici-populinaKleb. [II] | Populus simonii L. | ||
| Melampsora salicis-albae Kleb. [II] | Salix sp. | ||
| Puccinia arenariae (Schumach.) G. Winter [III] | Dianthus sp. | ||
| Puccinia coronata Corda [II] | Phragmites communis (Cav.)Trin. ex Steud | ||
| Puccinia hieracii var. piloselloidarum (Probst) Jørst. [III] | Hieracium pilosella L. | ||
| Puccinia phragmitis (Schumach.) Körn. [III] | Phragmites communis (Cav.)Trin. ex Steud | ||
| *Uromyces geranii (DC.) Lév. [II] | Geranium pratense L. Geranium robertianum L. | ||
| *Uromyces rumicis (Schumach.) G. Winter [II] | Rumex sp. | ||
| 11. | Stemphylium sarciniforme (Cavara) Wiltshire | Erysiphe alphitoides [mc] Erysiphe palczewskii [mc, ya, ma] | Q. robur C. arborescens |
| 12. | Tripospermum myrti (Lind) Hughes | Erysiphe alphitoides [mc, ya, ma] | Q. robur |
The greatest diversity of fungicolous fungi was found on Puccinia malvacearum, where three different fungi species were identified: Alternaria alternata, Epicoccum nigrum, and Cladosporium uredinicola (Table 1). Ampelomyces quisqualis has been reported for the first time ever in five species of the Erysiphales order fungi: E. deutzie, E. hedwigii, E. syringae-japonicae, E. urticae, and Uncinula adunca. In turn, the following three species turned out to be the new hosts for Sphaerellopsis filum: Melampsora larici-populina, Uromyces geranii, and Uromyces rumicis (Table 1).
All of these superparasites caused damage to the colonized structures of the host fungi, whereas Ampelomyces quisqualis colonized the mycelium, the conidial stage, the young, and, in the case of two species, the mature fruiting bodies of powdery mildew. Hyperparasites of the Pucciniales order: Sphaerellopsis filum and Cladosporium uredinicola, colonized various developmental stages – uredinia and telia. The formerproduced picnidia, whilee the letter – conidiophores with conidial spores. In turn, Clonostachys epichloë formed dark green sporodochia on the plate (stroma) and inhibited the development of the Epicloë typhina fruiting bodies (Table 1).
The remaining identified fungicolous fungi developed on the surface of the host fungi structures, which showed varying degrees of destruction. In the case of Stemphylium sarciniforme, the presence of structures was observed inside Erysiphe palczevskii appendages, whereas the absence of ascospores inside the fruiting bodies indicated the invasive nature of the relationship (Table 1).
. Discussion
Parasitic fungi are one of the important links of biocenosis. They are involved in various processes: from regulating host populations to developing new species, thereby triggering mechanisms of biological evolution, which makes them responsible for multiple processes related to biosphere functioning (Combes, 1999). The prevalence of parasites in a given environment depends primarily on the presence of host plants therein (Majewski, 1971). Hence, the most important task in the parasite’s life strategy is to meet a suitable host that will ensure its survival in various environmental structures (Mułenko, 1998). As a long-term interaction, the resulting ‘parasite–host’ system can be modified by the presence of the so-called fungicolous fungi, which can be symbionts, parasites, commensals, or saprobionts (Gams et al., 2004; Sun et al., 2019). Fungicolous fungi, which enter into close relationships with their hosts, can significantly affect the dynamics and size of their host populations in various ecosystems, including both natural and anthropogenic ones. The present study identified 12 representatives of fungicolous fungi, among which there were four hyperparasitic fungi: Ampelomyces quisqualis, Cladosporium uredinicola, Clonostachys epicloe, and Sphaerellopsis filum.
Typical hyperparasites found across the world include Ampelomyces quisqualis infesting powdery mildew (Erysiphales) (Manjunatha et al., 2020) and Sphaerellopsis filum parasitizing the developmental stages of rust fungi (Pucciniales) (Gams et al., 2004; Gordon & Pfender, 2012). Ampelomyces quisqualis was initially shown to be biotrophic and subsequently turned into a necrotroph (Gams et al., 2004). While developing inside the elements of the host’s thallus, the hyperparasite transforms them into its own spore-forming structures, thereby inhibiting the reproductive capability of the host fungus. In the present study, A. quisqualis colonized the mycelium, the conidial stage as well as young and mature fruiting bodies of powdery mildews. Literature data indicate that mature fruiting bodies are not colonized by a hyperparasite (Falk et al., 1995), whereas our previous research (Sucharzewska et al., 2012b) confirmed the presence of A. quisqualis inside mature fruiting bodies with well-developed appendages. Our recent investigations confirm the presence of the hyperparasite in mature fruiting bodies of Erysiphe vanbruntiana var. sambuci-racemosae on Sambucus racemosa and Erysiphe flexuosa on Aesculus hippocastanum, as well as conidial spores of A. quisqualis in fruiting bodies instead of asci and ascospores. The ability of the Ampelomyces genus fungi to colonize the thallus of numerous Erysiphales species and inhibit their reproduction has been applied in practice to develop a biological preparation AQ10 (Kiss et al., 2004).
Also, other fungi were detected on the mycelium and fruiting bodies of powdery mildew: Alternaria alternata, Aureobasidium pullulans, Cladosporium cladosporioides, and C. herbarum. Also, representatives of Alternaria, Botrytis, Candida, Cephalosporium, Cladosporium and Trichothecium may colonize powdery mildew thallus (Braun, 1995). Fungi of the genus Alternari are cosmopolitan and occurrs on plants as pathogens and endophytes and in the soil as saprophytes. According to DeMers (2022), all species of this genus exhibit at least mild pathogenicity, whereas a broad range of metabolites characteristic of the Alternaria section probably promote the ability of these fungi to establish various interactions with the host and feeding modes. In Poland, representatives of the genus Alternaria, including Alternaria alternata on the mycelium of Erysiphales, were noted by Adamska and Czerniawska (2010) and by Piątek (2003), who reported the occurrence of the Alternaria genus spores on the mycelium of Phyllactinia fraxini. In addition, Aureobasidium pullulans has been detected on the mycelium of two species: E. aphitoides and E. paleczewskii. They are cosmopolitan microorganisms, well documented in the literature and known as saprotrophs, isolated from the surface of plants, soil, water, rock surfaces, and man-made surfaces. Their antagonistic effects on plant pathogens, based on the mechanism of competition, as well as their capability to produce hydrolytic enzymes and antimicrobial compounds, have spurred great interest in the agricultural sector (Bozoudi & Tsaltas, 2018). The images taken by the scanning microscope (SEM) in the present study (unpublished data) revealed abundant mycelium of A. pullulans covering the mycelium and fruiting bodies of E. alphitoides and E. palczewskii. Fungi of the genus Cladosporium (identified as C. cladosporioides and C. herbarum) were detected in the present study on the Erysiphales mycelium and fruiting bodies. In Poland, this phenomenon has also been documented by Adamska and Czerniawska (2010). The literature works list 26 different species of this genus colonizing the thallus of other fungi across the world (Heuchert et al., 2005), with five species documented in Poland (Ruszkiewicz-Michalska & Mułenko, 2008). Both C. cladosporioides and C. herbarum were recorded in high numbers on the surface of various plant species (Mułenko et al., 2008); therefore, as polyphagous saprobionts, they can also colonize the thallus of various fungi species and at the same time exhibit antagonistic properties driven by the competition mechanism (Agrios, 2005). In the present study, these fungi colonized the surface of fruiting bodies, which were often empty, suggesting invasive contact between the fungi; however, this relationship needs to be further explored in detail. The study also revealed the presence of Stemphylium sarciniforme and Tripospermum myrtii, earlier noted on the mycelium of E. alphitoise colonizing Q. robur by Sucharzewska et al. (2011). Fungi of the genus Tripospermum are associated mainly with the matter of the aquatic environment (Karamchand & Sridhar, 2009). There are, however, several works describing the conidia of aquatic hyphomycetes in “unexpected places” (Magyar et al., 2005). Tripospermum myrtii was isolated as a saprobiont from, i.e., oak leaves (Adhicari & Manandhar, 1990), Acer saccarinum (after Mack, 2022), and dead branches (Seephueak et al., 2011). In the present study, it appeared together with another dark-filamentous species Stemphylium sarciniforme, whose structures were also found inside the Erysiphe palczewskii appendages. Likewise the fungi of the Alternaria and Cladosporium genera, these fungi can occasionally colonize Erysiphales’s thallus; however, establishing their relationship type would require further microscopic and molecular analyses. No other known fungicolous fungi, earlier documented on the thallus of Erysiphales, like Verticillium lecanii (Zimm.) Viègas, Acremonium alternatum Link. (Askary et al., 1998; Romero et al., 2003), Tilletiopsis sp. Derx (Urquhart et al., 1994), Sporothrix flocculosa Traquair Shaw & Jarvis (Elad, 1995), Phoma glomerata (Corda) Wollenw. & Hochapwel (Sullivan & White, 2000), Tilletiopsis minor and Trichothecium roseum (Gams et al., 2004), or Cladosporium uredinicola Speg (Dugan & Glawe, 2006), were found over the period of the study. The latter, which occurs mainly on various developmental stages of Puccinales fungi, colonizes also the representatives of Erysiphales: Phyllactinia angulata (E. S. Salomon) S. Blumer, and Phyllactinia guttata (Wallr.) Lév., or Phyllactinia fraxini (Dugan & Glawe, 2006; Heuchert et al., 2005; Ruszkiewicz-Michalska, 2010). In the present study, Cladosporium uredinicola was recorded on five species of Pucciniales, while in Poland, this parasite was recorded on 11 Pucciniales species (Dolińska et al., 2011; Mułenko et al., 2008), as well as on Taphrina pruni (Fuckel) Tul. (Ruszkiewicz-Michalska & Mułenko, 2008). In the present study, it was reported for the first time ever on Puccinia arenarie, P. glechomatis, and P. malvacearum.
Nevertheless, one of the most common parasites of the Pucciniales fungus is Sphaerellopsis filum (= Darluca filum (Biv.) Castagne), a biotrophic mycoparasite, establishing a close relationship with the host (Płachecka, 2005 and the literature cited therein). According to Kranz and Brandenburger (1981), it was recorded on 369 species belonging to 30 genera. In Poland, this common superparasite was detected in 53 rust species (Mułenko et al., 2008). In the present study, S. filum was reported on 11 species of rust fungi, including three new hosts. Molecular studies (Bayon et al., 2006) revealed genetic diversity within the Sphaerellopsis filum, and host-specific interactions of individual isolates (Nischwitz et al., 2005), which suggests the presence of more than one species. Other representatives of fungicolous fungi may also appear on Puccinales thallus, among which the species belonging to Cladosporium Link., Tuberculina Tode ex Sacc. (Mułenko et al., 2008) or Ramularia Unger (Bartkowska, 2007) have been most often mentioned in Poland. Other recorded species included: Gloeosporium roesteliicola, Fusarium avenaceum, F. graminearum, F. uredinicola, Hymenula spermogoniopsis and Trichothecium roseum (after Ruszkiewicz-Michalska, 2010) or Lecanicillium uredinophilum (Park et al., 2015). No such diversity of fungicolous fungi species has been noted in the present study, but instead, analyses revealed the presence of Alternaria alternata and Epicoccum nigrum, which are classified as polyphagous saprotrophs. Despite the extensive search, no fungi of the Tuberculina genus recorded in different places in Poland on various representatives of Pucciniales (Mułenko et al., 2008) were identified in the study area.
During the study, Clonostachys epichloë was reported at one site on the thallus of Epichloë typhina (Clavicipitaceae, Ascomycota). Epichloë typhina is an endophytic fungus found on many grass species around the world, forming a characteristic plate (stroma) around the leaf sheath, inhibiting the maturation of inflorescences (Lembicz et al., 2011; Song et al., 2016). Inside the stroma, the fungus forms numerous fruiting bodies with ascospores, which are the source of infection, whereas the presence of the superparasite reduces the inoculum of E. typhina (Górzynska et al., 2018). The present study also showed reduced numbers of fruiting bodies compared to the healthy stromata. Due to the inhibitory effect of Clonostachys epichloë on the development of Epichloe typhina, this hyperparasite has been applied in plant bio-protection. However, recent studies have also shown an adverse effect of its presence on the seed weight of Puccinellia distans. Therefore, increasing attention has recently been paid to the issues of the prevalence of this hyperparasite and its impact on the entire parasite host–hyperparasite system (Górzynska et al., 2023).
. Conclusions
The presented research results prove that the phenomenon of the prevalence of fungicolous fungi, including superparasites, is quite common in the urban environment, and the species diversity is due to the presence of host fungi. The thallus of fungi from the orders Erysiphales and Puccinales, on which the fungicolous fungi were recorded in the highest numbers, turned out to be a very good habitat for other fungi. When entering into various relationships, these fungi can affect the population number and structure of fungi parasitic to plants. Compared to natural environments, the anthropogenic environment represents a quite poorly understood biocenosis in terms of fungicolous fungi prevalence. The continuous introduction of new plant varieties generates the phenomenon of species diversity of fungi parasitic to plants and fungi colonizing them (hyperparasites), as confirmed by the present study results.