. Introduction
Rust fungi are pathogens causing diseases in plants cultivated and used by humans. As biotrophs, rusts are specially adapted to obtain nutrients from living plant cells. The name of these fungi is derived from the rusty color of the so-called summer spores (urediniospores). They are highly specialized pathogens with the ability to complete the entire morphologically and cytologically complicated life cycle on one (autoecious) or two (heteroecious) hosts. As pleomorphic fungi, they have the ability to form up to five different successive morphological stages of sporulation (basidiospores, spermatia, aeciospores, urediniospores, and teliospores) in a specific order (Majewski, 1979; Zhao et al., 2023). The Pucciniales order comprises one of the largest groups of phytopathogens within both the Fungi kingdom and rusts, which occur on mosses, ferns, and advanced monocots and dicots (Aime & McTaggart, 2021; Kirk et al., 2008; Zhao et al., 2023). Over half of the known rust species represent the Puccinia Pers. genus. The genus comprises approx. 4,000 species common in the world, occurring almost exclusively on angiosperms (Kirk et al., 2008).
Puccinia scirpi DC. (common club rush rust) was described in 1805 by Lamarck and de Candolle under this name and as Aecidium nymphoides DC., now considereda synonym of the previous name. Currently, the fungus Dicaeoma scirpi (DC.) Gray, introduced in 1821, and Aecidium nymphaeae Wallr. described as a new species in 1833, are regarded as P. scirpi (Index Fungorum, 2023). The fungus is macrocyclic and heteroecious rust noted on representatives of the Menyanthaceae and Cyperaceae families (Gäumann, 1959).
P. scirpi is a rare species in Poland. It was recorded on the first host, N. peltate, from only one locality over 100 years ago and has not been reported since then. In the telial stage, it was recorded at the same time from six localities (Majewski, 1979), one locality in 2012 (Czerniawska et al., 2012), and one in 2017 (locality from 2009) (Mazurkiewicz-Zapałowicz et al., 2017). To date, it has never been found in Poland in the uredinial stage; therefore, this is the first report of the fungus.
The Nymphoides genus (Menyanthaceae) comprises species of aquatic plants. The geographical range of Nymphoides peltata (S.G. Gmel.) Kuntze covers Europe from the Baltic States to the Iberian Peninsula and Asia from the Middle East through northern India, Siberia, Mongolia to China, Japan, and the Korean Peninsula. The species was also adventive to North America, where it spread (Lansdown, 2014; Ławicki & Marchowski, 2019). Throughout Europe, the plant is quite common in the valleys of large southern European rivers, but the number of populations is unknown. In some countries or their regions, the species is rare or very rare, while in several countries, it is classified as threatened on several national red lists (Lansdown, 2014). In Poland, the fringed water lily is a scarce species under legal protection and has the status of an endangered species. The plant occurs along rivers and estuaries. In 2001, the localities were not very numerous; at that time, there were about 45 existing, 40 declining, and several unconfirmed localities, but many of these no longer exist (Kłosowski, 2014; Zając & Zając, 2001). It inhabits mainly oxbow lakes, river bends, eutrophic lakes, and fish ponds (Ławicki & Marchowski, 2019).
The genus Schoenoplectus (Rchb.) Palla (Cyperaceae) is represented by over 70 species and subspecies of hydrophytes and helophytes (Mazurkiewicz-Zapałowicz et al., 2017; Smith, 2002). In Poland, four species of Schoenoplectus occur naturally, the most common of which is S. lacustris (L.) Palla (Mirek et al., 2002). S. lacustris is a common species throughout Poland (Zając & Zając, 2001).
Summarising the occurrence of both hosts of P. scirpi in Poland, it can be expected that, due to the occurrence of both plants close to each other, rust should occur slightly more frequently in the country; however, the sporadic occurrence of N. peltata reduces the incidence of rust. The fungus can complete its entire life cycle only in the presence of both hosts.
. Material and methods
Infected specimens of N. peltata and S. lacustris were collected in 2018 in Bolestraszyce Arboretum near Przemyśl in Poland. Microscope slides were prepared from air-dried specimens. Aeciospores, teliospores, and urediniospores were stained with cotton blue in lactic acid, heated, and observed under a light microscope. Microphotographs of fungal diagnostic structures of the species were taken with an Olympus digital camera SC180 and an Olympus BX53 light microscope.
Monographs by Majewski (1979) and Gäumann (1959) were used for rust identification. The nomenclature of the host plants follows the World Flora Online (WFO, 2023) The collected specimens are deposited in the herbarium of Maria Curie-Skłodowska University in Lublin (LBL).
. Results
Puccinia scirpi, the heteroecious rust species in the aecial (stage I) on N. peltata and uredinial (stage II), and telial (stage III) stages on S. lacustris were collected in 2018 in Poland from Bolestraszyce Arboretum near Przemyśl.
Taxonomy
Puccinia scirpi DC., in Lamarck & de Candolle, Fl. franç., Edn 3 (Paris) 2: 223 (1805) (Figure 1)
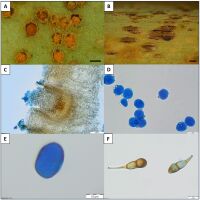
Figure 1
Puccinia scirpi (A) and (C) aecia with aeciospores on the leaf of N. peltata (LBL M–33129); (B) stem of S. lacustris with telia (LBL M–33126); (D) aeciospore (LBL M–33129); (E) urediniospores (LBL M–33128); (F) teliospores (LBL M–33127). Scale bars: (A) 0.2 mm; (B) 0.5 mm; (C) 100 µm; (D,F) 20 µm; (E) 10 µm. Photographs: (A), (C–F) U. Świderska; (B) M. Szewczyk.
Synonyms:
Aecidium nymphoidis DC., in Lamarck & de Candolle, Fl. franç., Edn 3 (Paris) 2: 597 (1805)
Dicaeoma scirpii (DC.) Gray, Nat. Arr. Brit. Pl. (London) 1: 542 (1821)
Aecidium nymphaeae Wallr., Fl. crypt. Germ. (Norimbergae) 2: 255 (1833)
Description:—Aecia epiphyllous, cup-shaped, up to 0.5 µm in diameter. Peridium cup-shaped, with a folded margin; peridium cells in regular rows, external wall up to 7 µm thick, surface densely and coarsely verrucose, inner wall up to 4 µm thick, surface densely and finely verruculose, 22–34 × 17–23 µm. Aeciospores oblate, catenulate, 15–20 × 19–23 µm, wall up to 1.4 µm thick, usually verruculose. Uredinia elongated, up to 0.5 mm long, long covered by a vesicularly raised epidermis. Urediniospores irregularly ovate or elliptical, 20–25(–28) × l4–21 µm; yellowish, 1.5–2 µm thick, covered by fine spikes. Germ pores 2, ± equatorial. Telia elongated, up to 2 mm long, covered by a persistent brown epidermis, after tearing, they are black, dust-free. Teliospores (sub-)clavate, usually rounded at the apex, less often irregularly flattened or pointed, tapering at the base, constricted at the septum, 33–58 × 13–23 µm. Wall brown-yellow, smooth, thickened in the distal cell up to 7–10 µm. Germ pore indistinct. Pedicel/stalk up to 62 µm long, persistent, separated from the base of the teliospores by a diagonal wall.
Examined collections:—On Nymphoides peltata (S.G.Gmel.) Kuntze (Menyanthaceae), stage I. POLAND. Subcarpathian Voivodeship: Bolestraszyce Arboretum n. Przemyśl, 2 June 2018, leg. R. Rozwałka, det. A. Wołczańska (LBL M–33129). On Schoenoplectus lacustris (L.) Palla (Cyperaceae), stage II, III. POLAND. Subcarpathian Voivodeship: Bolestraszyce Arboretum n. Przemyśl, 2 June 2018 (last year’s shoots), leg. R. Rozwałka, det. A. Wołczańska (LBL M–33127); 10 August 2018, leg. R. Rozwałka, det. A. Wołczańska (LBL M–33128); 10 October 2018, leg. R. Rozwałka, det. A. Wołczańska (LBL M–33126).
Literature data:—On N. peltata, Limnanthemum nymphoides (hosts need revision). POLAND. Motława River in Gdańsk (Majewski, 1979). On S. lacustris. POLAND. Wolin Island, Kołobrzeg, near Legnica and Ścinawa, vicinity of Cracow and Międzyrzec Podlaski (Majewski, 1979); Wasosze Lake (West Pomerian province) (Czerniawska et al., 2012); Płociczno Lake (Mazurkiewicz-Zapałowicz et al., 2017).
General distribution:— Europe, North Africa; Asia, North America, New Zealand.
. Discussion
P. scirpi is a dioecious and heteroecious rust, usually with members of Nymphoides as aecial hosts and representatives of Schoenoplectus (= Scripus) as uredinial and telial hosts. The fungus has also been noted in all stages of sporulation on representatives from a few other host genera (Farr & Rossman, 2021).
On N. peltata, the rust fungus has been reported from Bulgaria, Lithuania, Norway, and the United Kingdom, in addition to Poland. On the members of the Nymphoides genus, the fungus has also been reported worldwide on N. forbesii (probably N. forbesiana) and N. indica subsp. occidentalis (both from Uganda), N. grayana (from Cuba), and Nymphoides sp. (from the USA – Florida) (Farr & Rossman, 2021; Soares et al., 2006). The fungus has been noted on S. lacustris in Bulgaria, Denmark, France, Germany, Greece, Lithuania, Norway, Poland, Romania, Spain, Sweden, the United Kingdom, Ukraine, Puerto Rico, China, and Iran (Farr & Rossman, 2021). It is known to infect a few other Schoenoplectus species, i.e., S. litoralis (India, Iran, and Pakistan), S. tabernaemontani (the Dominican Republic, Japan, Lithuania, New Zealand, Puerto Rico, Thailand, the Virgin Islands, and the West Indies), S. triqueter (China, Japan, Taiwan), and S. validus (New Zealand and Thailand) (Farr & Rossman, 2021; Soares et al., 2006; Wu et al., 1982).
The fungus has been reported from eight localities in Poland (Wolin Island, Kołobrzeg, Jakuszów near Legnica, Orzeszków near Ścinawa, surroundings of Cracow and Międzyrzec Podlaski, Wasosze Lake, and Płociczno Lake) on S. lacustris in the telial stage (stage III) (Czerniawska et al., 2012; Majewski, 1979; Mazurkiewicz-Zapałowicz et al., 2017). In the uredinial stage (stage II), the fungus has not been published from Poland to date. In the aecial stage (stage I), the species has been noted only in Gdańsk (on the Motława river) (Majewski, 1979), and this is the second report in this stage since the end of the 19th century in Poland. The rare occurrence of N. peltata may be associated with the sporadic, although the dispersed occurrence of this host in its natural habitats (Gdaniec, 2010; Mazurkiewicz-Zapałowicz et al., 2017).


