. Introduction
Lobaria pulmonaria is widely recognized as a key lichen species characterizing old-growth forests (Brodo et al., 2001; Jüriado & Liira, 2010; Kondratyuk et al., 1998; Motiejūnaitė et al., 2004; Nadyeina et al., 2014). Given the research by Goward (1994), Werth (2001), and Werth et al. (2007) it can be assumed that as in the other species with an extensive geographical range (Brodo et al., 2001; Widmer et al., 2012) its habitat specialization is increasing as well as its bioindicative role as a stenotopic species in the gradient of progressive climate continentalism (Nadyeina et al., 2014). The history of the forest ecosystem, its fragmentation, and even the way it is used affects the genetic variability of the L. pulmonaria population, the continuity of propagation, the success of dispersion, the vitality and may determine the survival of this species (Bianchi et al., 2020; Brunialti et al., 2015b; Öckinger et al., 2005; Scheidegger & Werth, 2009; Zoller et al., 1999). The key habitat factors for its occurrence seem to be: stable, high air humidity, and diffused sunlight, which prevent long periods of thallus drying (Gauslaa & Solhaug, 1999; Khanov & Pshegusov, 2021). Despite many threats resulting from direct or indirect anthropopressure, the European population of L. pulmonaria is still relatively numerous and genetically diverse (Widmer et al., 2012), although there are many examples of reduction and disappearance of its regional or local ranges (Fałtynowicz, 2003; Farkas & Lőkös, 1998; Jüriado & Liira, 2010; Nascimbene et al., 2016). L. pulmonaria shares specific microclimatic requirements with other rare lichen species sensitive to forest disturbances whatpredispose this easily recognizable macrolichen to the role of an umbrella species (Bianchi et al., 2020; Nascimbene et al., 2010; Nilsson et al., 1995; Scheidegger & Werth, 2009). Umbrella species are considered to be species with high requirements, which, while providing a sufficient protected area, contribute to the protection of co-occurring species and help identify species-rich areas on a large geographical scale (Caro, 2003). The presence of larger and more viable populations of L. pulmonaria corresponds with more lichen species richness in general, more macrolichen diversity, and more abundance of rare lichen species and cyanolichens, as well as a species composition (Campbell & Fredeen, 2004; Nascimbene et al., 2010). The decrease in the area of key habitats for L. pulmonaria and the subsequent downward trend in the number of the species prompted the governments of several countries to place it under legal protection and grant it the status of special concern (e.g., Khanov & Pshegusov, 2021; Scheidegger & Clerc, 2002). The legal protection of L. pulmonaria was applied among others in Hungary, Germany, Estonia, Ukraine, and Croatia. In France, Russia, and the United Kingdom it is only under regional protection (Paoli et al., 2019). This approach has also been used in Poland for almost 20 years, where, in addition to the classic species protection that prohibits the destruction of thalli and habitats, this species is also subject to zone protection (RME, 2014a; Ryś, 2007). Despite the official determination of the maximum size of the protection zone, its optimal size for the preservation of L. pulmonaria sites, as well as for 10 other stenotopic forest lichen species, is a debatable and unexplored issue in Poland. Changing the size of this protection zone from an area limited by a radius of 100 m (RME, 2004) to a zone with a radius of up to 50 m (RME, 2014a) raises the question of its legitimacy, especially in the context of the umbrella role of L. pulmonaria for the preservation of stable habitats and the resulting diversity of co-occurring species. It is no secret that usually strictly protected zones created under EU law for various protected objects, constitute a significant limitation of economic functions in forests (Ryś, 2007). They become particularly controversial for the land managers in those fragments of forests that have still retained their natural character, manifested by the abundance of endangered and protected species, and require many hectares of zone protection. The conflict between nature protection and the economy has a centuries-long tradition, but the basis for administrative decisions taken to protect natural resources should be scientifically justified, especially since it is the awareness of officials that determines the establishment and size of a protection zone. We were looking forward to such justification when undertaking research in the Polish Carpathians on the relationship between the size of protection zones for L. pulmonaria and the diversity of lichen species in the context of the discussion on the proposed changes to the law on the protection of the lichen species (Fałtynowicz, 2021a, 2021b, 2021c).
The aim of the study was to obtain scientific data that would enable the assessment of the validity of changes in the legal protection of Lobaria pulmonaria in the context of the corticolous lichen diversity conservation, including stenotopic, endangered and legally protected species. We hypothesized that the size of the L. pulmonaria protection zone is important for the conservation of a greater diversity of corticolous lichens in the Polish Carpathians.
. Material and methods
Study area as a background for the occurrence of Lobaria pulmonaria
Field lichenological research was carried out in the years 2015–2020 in the area of Gorce National Park, the wildest part of the Gorce Mts in the Polish Western Carpathians and the Stuposiany Forest District in the Bieszczady Zachodnie Mts, a part of the Polish Eastern Carpathians (Figure 1). The choice of research areas was based on the contemporary occurrence of L. pulmonaria in the Polish part of the Carpathians (Czarnota, 2000; Kościelniak, 2013; Ryś, 2007; Tuchowski, 2022; Wąsik, 2016) and the maximum elimination of anthropogenic factors that could affect the composition of the lichen biota in the extensive zones around L. pulmonaria sites, which were legally protected areas, or in the state forests were not subject to intensive economic use in the last few decades. In view of such assumptions, the natural dynamics of forest stands resulting from the natural mortality of trees seemed to be the almost sole reason for the formation of the contemporary biota of lichens accompanying L. pulmonaria in these areas.
Figure 1
Localities of field lichenological research on the background of the Carpathians (A) and the maps of Gorce National Park (B) and Stuposiany Forest District (C). The meaning of the colours used in Figure 1B and Figure 1C: light green - wooded area, dark green - strictly protected area, red - sites of the researched protected zones of Lobaria pulmonaria.
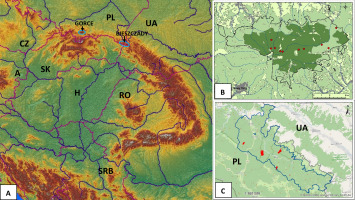
Gorce National Park, erected in 1981, covers a large area of natural and somewhere even primeval mixed lower-belt forests mostly represented by various forms of the Carpathian beech forest Dentario glandulosae-Fagetum. In the best-preserved parts of such forests, but also in parts used for logging, L. pulmonaria has been found throughout the last century (Czarnota, 2000; Czarnota et al., 2005; Glanc, 1960; Motyka, 1930). The most numerous sites of this species (18), found as a result of intensive research on the Gorce lichen biota, were recorded at the turn of the 1950s and 1960s (Czarnota et al., 2005; Glanc, 1960). About half of them survived until the end of the century, and a few new ones were found in the past decade (Tuchowski, 2022; Wąsik, 2016). Certainly, most of the sites were lost for natural reasons as a result of the death of host trees (Czarnota, pers. inform.). In 2022, L. pulmonaria was recorded in the Gorce Mts at 10 sites, inhabiting ancient, sometimes heavily decayed beeches with a diameter of 57–97 cm DBH with small thalli dominating in this population and yet showing signs of degeneration (Tuchowski, 2022). There was no legal zonal protection around these sites due to their strict protection in the national park.
The Western Bieszczady Mts is a vast complex of medium-high mountains on the border of Poland, Ukraine, and Slovakia (1150 km2; Solon et al., 2018), with the remains of beech and sycamore forests that have retained their ancient character. It was evidenced already in the 1950s by numerous records of many species of stenotopic macrolichens, representing genera such as Cetrelia, Leptogium, Lobaria, Nephroma, Parmotrema, Usnea (Glanc & Tobolewski, 1960). The most valuable natural parts of these forests have been protected within the borders of the Bieszczady National Park established in 1973, but equally valuable areas of old deciduous forests Dentario glandulosae-Fagetum are located among others in the managed state forests of the Stuposiany Forest District, in the belt bordering the national park. As the last observations of the authors have shown, data from the Regional Directorate for Environmental Protection in Rzeszów, the government body responsible for nature conservation in this region of Poland, and previous inventories of L. pulmonaria sites (Ryś, 2007), locally in this area this species still occurred frequently and numerously. Its thalli often exceed 50 cm in diameter and sometimes develop fruiting bodies (Kościelniak & Betleja, 2015). The protection zones designated there in the last few years now cover a total area of several dozen hectares, with some of them grouping up to several dozen old beeches and sycamores of more than 100 cm DBH inhabited by L. pulmonaria. From the multitude of sites, five were selected for our study (Figure 1C).
Characteristics of studied sites
All studied sites in the Bieszczady Mts and Gorce Mts were located in the area covered with the Carpathian beech forest Dentario glandulosae-Fagetum, differing, however, in both ranges, since in Bieszczady Mts sycamore besides beech often plays the role of the main forest-forming species while in Gorce Mts, the higher altitude, the share of Norway spruce is much larger. All sites were chosen in old-growth forests (Table 1) with more or less numerous trees of younger generations. Individual sites differ in the spatial and age structure of the stands, shaped in recent decades mainly by the natural processes of dieback and recruitment.
Table 1
Location of sites and habitat characteristics of studied forest areas in Polish Carpathians. Abbreviations: G1–G10 – sites located in Gorce Mts; B1–B5 – sites located in Bieszczady Mts; Fs – Fagus sylvatica; Aa – Abies alba; Pa – Picea abies; Ap – Acer pseudoplatanus. No. of forest division and age of oldest tree among dominant tree species according to on-line version of Bank of State Forests data (https://www.bdl.lasy.gov.pl/portal/mapy; last access 15.03.2023). Tree species mentioned as first have the main share in the stand composition.
Most sites of L. pulmonaria were located on the cooler, northern, and eastern exposures of slopes, if in the lower parts of the mountains, then usually in the depressions of the river valleys, stabilizing humid microclimate. In the Gorce Mts, the sites were located on ridges and slopes, but higher than in Bieszczady Mts, in the zone of more abundant and frequent precipitation, including snow.
Methods
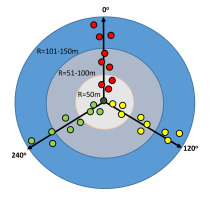
The diversity of epiphytic lichens was assessed at 15 sites, established in the form of a circular area of 7.07 ha each, the central point (CP) of which was a previously selected tree inhabited by Lobaria pulmonaria thalli. Trees were then selected at this site to prepare a list of species. Beech trees (Fagus sylvatica) and less frequently sycamore trees (Acer pseudoplatanus), reaching a diameter at breast height of d1.3 ≥ 40 cm, were selected, growing along three designated transects, radiating from the central point in accordance with the azimuth of 0°, 120° and 240°, and the host tree for L. pulmonaria. The transects were divided into three zones depending on the distance from the central point, named (i) R = 50 m, (ii) R = 51–100 m, and (iii) R = 101–150 m, corresponding respectively to a radius of 50 m, a radius section in the range of 51–100 m and a radius section in the range of 101–150 m. 28 trees were planned to be examined at each site (Figure 2). Exceptionally, when there were no trees of both species meeting the DBH criteria, beeches with a lower diameter at breast height were selected for sampling; however, in the absence of both phorophytes within 20 m of the transect, no sample trees were selected. Based on data for the three concentric zones, the diversity of lichens was also determined for zones with a radius of 100 m and 150 m, further named as zone R = 100 m and R = 150 m, respectively.
Figure 2
Scheme showing the distribution of sample trees in three analyzed concentric zones around the host-tree of L. pulmonaria.
Species difficult to identify in the field were collected for laboratory analysis where a standard spot test reaction with ethanol solution of paraphenylenediamine, KOH, and NaClO (in the commercial form of ACE) and UV irradiation were applied, if necessary. Sterile thallus fragments were identified by TLC thin-layer chromatography in eluent C (Orange et al., 2001). Species nomenclature was adopted from Printzen et al. (2022); for several species not listed in this paper, nomenclature from Index Fungorum (https://www.indexfungorum.org; accessed 12 January 2023) was used. Due to the ease of field mistakes in identifying some species recently described and macroscopically similar to each other, the research was limited to distinguishing collective taxa Micarea micrococca s.lat. (except for the characteristic species M. byssacea (Th. Fr.) Czarnota, Guzow-Krzem. & Coppins) and Parmelia saxatilis s.lat. (although P. serrana A. Crespo, M.C. Molina & D. Hawksw was also identified on single trees in Bieszczady Mts).
The obtained results were compiled in a database, distinguishing in each concentric zone threatened species that are red-listed in Poland (Cieśliński et al., 2006), lichen indicators of East-Central-European primeval forests (Motiejūnaitė et al., 2004) and species legally protected in Poland (RME, 2014a). To visually assess the similarity in lichen communities between sites and concentric zones, the detrended correspondence analysis (DCA) from the CANOCO v. 5 package was used, implementing the ‘detrending by segments’ option. The measure of species abundance was the number of trees inhabited by a given species in a given zone at a given site.
The significance of differences in lichen species diversity (the number of lichen species) between the sites and between the three zones (R = 50 m, R = 51–100 m, R = 101–150 m) around the tree-site of L. pulmonaria was tested by the non-parametric Kruskal–Wallis test. Dunn’s post hoc multiple comparison test was used to examine which zones differed significantly from each other. The homogeneity of variance was tested using the Brown–Forsythe test. The normality of the distribution of the random variable was tested using the Shapiro–Wilk test. The dependence of lichen diversity on zone size was examined using Spearman’s rank correlation coefficient (rs). The biodiversity and structure of lichen communities were assessed using the Shannon-Wiener index (H) and the Pielou evenness index (J). The statistical analyses were performed using STATISTICA v. 13.1 at the significance level of α = 0.05.
. Results
A total of 157 species of lichenized and six species of lichenicolous fungi were found on 371 examined trees in zone R = 150 m, including 132, 116, 120, and 151 species within concentric zones R = 50 m, R = 51–100 m, R = 101–150 m, and R = 100 m (Table 2).
Table 2
The list of lichenized and lichenicolous fungi found in concentric zones around 15 host-trees of Lobaria pulmonaria in Polish Carpathians. Abbreviations: R – radius around the host-tree of Lobaria pulmonaria; N – number of examined trees in each concentric zone; L – number of trees hosting particular lichen species in each concentric zone; F – frequency; * – species found only in Gorce Mts; ** – species found only in Bieszczady Mts; # – lichenicolous fungus.
On average, 58 species of lichens were recorded at the study sites, and the number ranged from 42 species at the B4/Tarnawa site in the Bieszczady to 84 at the G10/Jaworzyna Kamienicka II site in the Gorce Mts. In eight out of 15 cases, the concentric zone up to 50 m was characterized by a greater number of species (on average 40 species) than at the least one further zone, ranging from 23 at site B3/Procisne in the Bieszczady to 55 species at site G3/Spaleniec in Gorce Mts. The R = 101–150 m zone was characterized by a slightly higher average number of species than the R = 51–100 m zone, but the difference was insignificant (Table 3).
Table 3
Number of lichen species in examined concentric zones around the Lobaria pulmonaria host-trees in Polish Carpathians. Abbreviations: R – radius around the host-tree of L. pulmonaria; G – sites in Gorce Mts; B – sites in Bieszczady Mts.
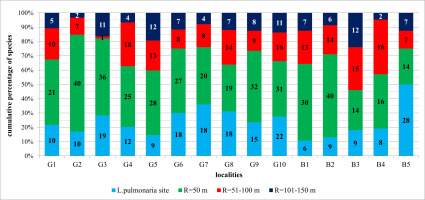
The share of the number of species in the zone R = 50 m in relation to the number of all species found at the site ranged from 46% to 85% (Figure 3), an average of 68.6%. The lowest share was recorded at the site B3/Procisne. Enlarging the concentric zone to a radius of 100 m resulted in an increase in the number of species from 2% at site G3/Spaleniec in Gorce Mts to as much as 38% at site B4/Tarnawa. The share of the number of species occurring in the zone R = 100 m in the scale of the studied sites in the Polish Carpathians increased significantly to the level of 76–97%, an average of 88.2% (Figure 3).
Figure 3
Contribution of lichen species in examined concentric zones around the Lobaria pulmonaria host-trees in the Polish Carpathians. For an explanation of research plots designations see Table 3.
Species diversity and structure of lichen communities measured by the values of Shannon-Wiener index (H) and Pielou’s evenness index (J), respectively, despite differences in the absolute number of species, were similar in all concentric zones, both considered separately for the sites in Gorce Mts, and in Bieszczady Mts, and jointly in both Carpathian regions (Table 4). This was also confirmed by the insignificant result of the analysis of variance (Kruskal–Wallis test: Gorce Mts p = 0.1811, Bieszczady Mts p = 0.6472, Carpathians p = 0.8246) in contrast to the significant one, showing the difference in the number of species between at least two sites within and between each of the mountain ranges. As the analysis of Dunn’s multiple comparisons showed, the greatest differences in the number of species among the 15 analyzed sites were found at the sites of G9/Jaworzyna Kamienicka I and G10/Jaworzyna Kamienicka II in Gorce Mts vs. B4/Tarnawa in Bieszczady Mts. The lichen species diversity of the site Jaworzyna Kamienica I significantly differed moreover from the diversity of more than half of the other studied sites (Table 5).
Table 4
Values of Shannon-Wiener index (H) and Pielou’s evenness index (J) for examined concentric zones around Lobaria pulmonaria host-trees in the Polish Carpathians. R – radius around the host-tree of L. pulmonaria.
Table 5
Results of the analysis of Dunn’s multiple (two-tailed) comparisons between the number of lichen species found on each examined tree at each sampled site located in the Polish Carpathians. Only significant differences at p < 0.05 level have been showed (for more precise results, see Table S1).
Spearman’s rank correlation analysis (rs = 0.66) indicated the existence of a statistically significant, strong, positive relationship between the size of the protection zone and the number of species.
In addition to the differences in the number of species between individual sites, the species composition of lichen communities also showed differences, and they were particularly visible between the two examined ranges of the Polish Carpathians (Figure 4). This was influenced not only by the effect of local habitat conditions, but also by the presence of many species recorded in the study zones in only one or the other mountain range (see Table 2) resulting from regional biological diversity in the Carpathian chain. Taking into account only the list of taxa exclusive to the region found in the research, the following were recorded only in Gorce Mts so far: Bacidia biatorina, Micarea synotheoides, Puttea margaritella, Schismatomma pericleum, and in Bieszczady Mts: Bacidia laurocerasi, Biatora mendax, Chaenotheca chlorella, Inoderma byssaceum, Opegrapha vermicellifera, Parmelia submontana, Parmelina pastillifera and Pertusaria flavida (Table 2).
Figure 4
Detrended correspondence analysis (DCA) diagram visualizing differences in the lichen species diversity related to examined circular zones around Lobaria pulmonaria host-trees in the Polish Carpathians. The two axes explain together 37.03% of the total variation in species composition (27.33% axis 1). Abbreviations: B1–B5 – localities in Bieszczady Mts, G1–G10 – localities in Gorce Mts (designations of the examined sites correspond to those given in Table 3).
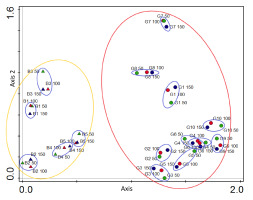
Within individual sites, the similarity of lichen species compositions in subsequent zones is high, which means that in most cases shown by the DCA correspondence analysis, they grouped into separate assemblages (Figure 4). This proves, together with the equalized Shannon-Wiener diversity indices (H), the relative biological homogeneity of habitat patches selected by Lobaria pulmonaria for colonization. At the same time, there are differences between the sites within the same mountain range. The most different lists of species within the Bieszczady Mts concern sites B1 and B3 and sites G7, G8, and G1 in the Gorce Mts.
A total of 25 species of primeval forest lichen indicators were found, of which six occurred only in the Gorce Mts and seven only in the Bieszczady Mts. Many species were recorded in small amounts, some only once. It has been shown that the zone up to 50 m protected the vast majority of lichens that preferred the ecological conditions of old-growth forests (approx. 70%), as well in the case of both Carpathian ranges considered separately as jointly (Figure 5). However, this zone did not protect seven species.
Figure 5
Lichen indicators of old-growth forests (Motiejūnaitė et al., 2004) found within the concentric zones around the Lobaria pulmonaria host-trees. Abbreviations: A – Polish Carpathians; B – Gorce Mts; C – Bieszczady Mts; * – species found only in Gorce Mts; ** – species found only in Bieszczady Mts; R – radius around the host-tree of L. pulmonaria. Pie charts show the percentage of new species in subsequent concentric zones.
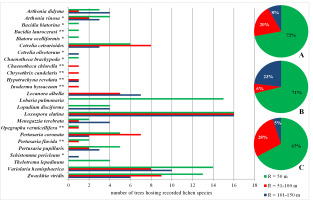
The extension of the zone to R = 100 m also increased the share of lichen species protected in Poland from 77% to as much as 95%. In the case of Gorce Mts, this increase was insignificant and amounted to only 6%, but in the case of Bieszczady Mts, the extension of the protection zone increased the number of protected species by as much as 1/3.
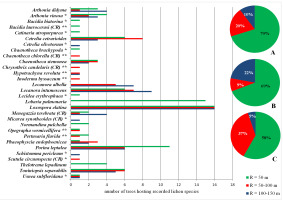
A total of 30 endangered and critically endangered species from the Polish red list of lichens were found, of which 10 were only in Gorce Mts and seven only in Bieszczady Mts. As in the case of the primeval forest indicators, the zone up to 50 m protected most of these species in each of the mountain ranges separately (69% in Gorce Mts and 58% in Bieszczady Mts) and jointly (70%); nine species were not found in any of these bands in the zone up to 50 m. The extension of the zone to R = 100 m significantly extended the protective umbrella for this group of red-listed lichens overall by as much as 20% and in Bieszczady Mts alone by as much as 37%. It is worth noting that out of the six identified critically endangered species, only two were found in the smallest zone (Figure 6).
Figure 6
Endangered (EN) and critical endangered (CR) lichen species in Poland (Cieśliński et al., 2006; IUCN criteria) found within the concentric zones around the Lobaria pulmonaria host-trees. Abbreviations: A – Polish Carpathians; B – Gorce Mts; C – Bieszczady Mts; * – species found only in Gorce Mts; ** – species found only in Bieszczady Mts; R – radius around the host-tree of L. pulmonaria. Pie charts show the percentage of new species in subsequent concentric zones.
. Discussion
Zonal protection as a form of biodiversity conservation
The term ‘biological diversity’ became widely known with the adoption by the international community of the Convention on biological diversity (CBD) during Earth Summit in Rio de Janeiro in 1992. Protection of biological diversity at three levels of complexity: genetic, species and ecosystem, then became one of the main directions of nature protection in the world. The implementation of this model in practice resulted in, among others, territorial protection legislation for particularly valuable species with specific habitat requirements (e.g., Aguilar-Støen & Dhillion, 2003; BiH Regulation, 2009; Hufty & Muttenzer, 2017; Prip, 2018; UNEP, 1992; Zimmermann et al., 2003).
Zonal nature protection has also gained recognition and application in Poland (RME, 2004, 2014a, 2014b, 2016), especially in relation to threatened birds (e.g., white-tailed eagle, golden eagle, black stork) and mammals in the breeding season (e.g., Jakubiec & Zyśk-Gorczyńska, 2012; Loch, 2010; Mikusek, 2012; Mizera, 2006; Zawadzki et al., 2020, 2022), but also to lichenized fungi (Ginszt et al., 2022; Kościelniak & Betleja, 2015; Kubiak et al., 2017; Ryś, 2007). The intention of using this form of protection is to protect the breeding and rearing area by conserving the habitat and ecological conditions that are conducive to the presence and spread of the protected species. Polish legislative acts define measurably the maximum size of zone but do not precisely define the methods of use within them, leaving the possibility of creating the zone and the selection of the most beneficial action to the administrative services appointed for this purpose. In most cases, creating a zone is understood as a form of passive protection, recognizing that this method of protection best meets the requirements of the protected species. As the zones are created for forest species, their areas, often many hectares long (e.g., time zones used during the breeding season of the black stork can reach 78.5 ha), constitute limitations in the economic use of forests, at least temporarily excluding these areas from harvesting and breeding treatments. The maximum size of these zones seems to be more or less speculation by experts, which in Poland has only recently been subject to scientific verification (Zawadzka et al., 2017). It is no different with regard to the size of zones proposed for lichens. Therefore, the legal reduction of the radius of the zone from 100 m, which was in force in 2004–2014, to the current radius of 50 m, was supposed to be a speculative compromise between the need for species protection and the economic viability of forest management. This action, like the previous one, requiring the creation of zones with a radius of 100 m, was purely administrative in nature and was not supported by any empirical data that would justify both the need to protect the lichen species itself and its umbrella role in the context of zonal protection of stenotopic species. The research results presented in our work are the first to address this issue, and their application nature can be used to verify the correctness of official orders and restrictions. In view of the ongoing discussion in recent years on the legitimacy of uniform patterns of species protection throughout Poland and the reasonableness of restrictions caused by zonal protection in general (Fałtynowicz, 2021a, 2021b, 2021c), undertaking this research topic on the eve of subsequent legislative changes becomes particularly important.
Lobaria pulmonaria as a species predisposed to the role of an umbrella species
Among the 11 species of lichens requiring zonal protection under Polish law, only Lobaria pulmonaria has been the subject of many studies around the world, specifying its biology, phylogeography, dispersion, and ecological requirements (e.g., Di Nuzzo et al., 2022; Rubio-Salcedo et al., 2015; Scheidegger et al., 2012). Its undoubted umbrella role was mainly reduced to treating this species as a forest stenobiont, requiring long ecological continuity of the nearest environment and the presence of natural habitats (Campbell & Fredeen, 2004; Nascimbene et al., 2010, 2013). The determining factors seem to be, above all, the high forest air humidity, the temperature, and the level of precipitation (Muir et al., 1997), which in the time of global climate change as continental influences increase, are becoming increasingly important for this species (Khanov & Pshegusov, 2021; Nascimbene et al., 2016).
Lobaria pulmonaria usually inhabits old-growth forests, which is why it is perceived as an indicator of forest ecological continuity (e.g., Brunialti et al., 2015b; Whittet & Ellis, 2013), which reacts negatively to changes in the stand structure caused by forest management and human activity (Di Nuzzo et al., 2022; Edman et al., 2008; Jüriado & Liira, 2010; Otálora et al., 2011). The sensitivity of this species and its high ecological requirements make it a good indicator of key habitats for relic and rare epiphytic lichens in autochthonous or ancient forests (Brunialti et al., 2015a; Motiejūnaitė et al., 2004; Nilsson et al., 1995; Paoli et al., 2019).
It also inhabits managed forests, but its frequency is lower than in primeval forests. The reason for this is forest fragmentation, leading to disturbances in the naturalness of natural processes (Scheidegger et al., 2012). The lack of age and structural continuity of stands shaped by anthropogenic disturbances is an obstacle to the sexual reproduction of heterothallic L. pulmonaria, leading to a decrease in its genetic diversity and imposing clonality (Singh et al., 2015). However, the effectiveness of vegetative propagation, which is the main method of dispersal of the species, is limited to short distances (Ronnås et al., 2017; Werth et al., 2014) and is possible only several dozen years after establishing a symbiotic association (Scheidegger & Goward, 2002), during which maintaining optimal microhabitat conditions is sometimes impossible. Larger gaps in stands cause negative changes in humidity and light ratios (Bianchi et al., 2020; Eaton & Ellis, 2014; Hilmo et al., 2012; Paoli et al., 2019; Walser et al., 2004), factors determining the development of L. pulmonaria (e.g., Di Nuzzo et al., 2022; Gauslaa et al., 2007).
In Poland, ancient forests currently cover a relatively small area. Large forest complexes of a primeval nature in north-eastern Poland deserve special mention, of which the Białowieża Primeval Forest is the most famous. The naturalness and compactness of the forest complexes of this area, as well as the large share of old trees, favor the occurrence of rich corticolous and wood-inhabiting lichen biota, including rare forest relics (Cieśliński & Czyżewska, 2002; Czerepko et al., 2021; Golubkov et al., 2011; Kukwa et al., 2008). L. pulmonaria was recorded in these areas at almost 300 sites, and in the area of the Białowieża and Augustów Forests also fruit-bearing individuals rarely were found in Poland (Matwiejuk, 2015; Matwiejuk & Zbyryt, 2013; Ryś, 2007).
In southern Poland, fragments of the Carpathian Primeval Forest preserved to this day are valuable complexes. The largest of them have survived in the Bieszczady Mts in the area of the Eastern Carpathians, where the natural and sometimes almost primeval nature of the forest makes it possible for many lichens, of which about 50% are endangered species at the national level. There is also the largest mountain population of L. pulmonaria in Poland (Kościelniak, 2013; Kościelniak & Betleja, 2015), including individuals forming large thalli, sometimes also with apothecia. At least 170 sites of this species were recorded in the Stuposiany Forest District, where the study was carried out as a part of this research (RDOŚ; letter reference WSI.402.215.2020.RW.2 of 16.09.2020).
Fragments of the ancient Carpathian forests have also been preserved in the Western Carpathians, e.g., in the Gorce range, which is a current refuge for over 500 species of lichens (Czarnota, 2010). Nevertheless, the Gorce population of L. pulmonaria currently occupies only 10 sites located in the strict protection area of the Gorce National Park, both in those parts of the forest that have been shaped almost exclusively by natural processes for decades and in those that were covered with a form of protection only after the establishing of the national park. L. pulmonaria thalli, which show signs of degeneration, are usually small, reaching a maximum size of several centimetres in individual cases. However, no fruiting individuals were found despite the documented long-term growth of some of them (Czarnota, unpublished).
The importance of the size of the protection zone for maintaining the species diversity of lichens
At the studied sites in the Polish Carpathians, in the widest zones R ≤ 150, the average number of epiphytic species was 58 (Table 3), with the differences between the sites being almost double in extreme cases. Many sites in such expressed species diversity showed statistically confirmed differences both on local and regional scales (Table 5, Figure 4). It could be related to the age of the oldest trees in the stand (Table 2), and thus also to their thickness and indirectly to the origin of the ecosystem, which has been repeatedly demonstrated in other studies (Dymytrova et al., 2014; Fritz & Brunet, 2010; Fritz et al., 2009; Hofmeister et al., 2016; Zemanová et al., 2017). Older trees may provide a habitat for more epiphytic species due to the larger surface of the substrate, a more diverse structure of the bark, and a greater number of microhabitats (Kubiak & Osyczka, 2020; Ranius et al., 2008). Undoubtedly, the studied mountain ranges of the Polish Carpathians are characterized by a different lichen biota expressed not only in the number of species (compare Bielczyk, 2003 vs Kościelniak, 2013) but also in the communities, which was clearly shown by the DCA analysis (Figure 4). This is facilitated, among others, by geographic regionalism resulting in different habitat conditions of the Western and Eastern Carpathians, which is also visible in relation to other components of the biocoenosis (e.g., Piękoś-Mirkowa & Mirek, 2003).
The average number of lichen species in the zone R = 50 m (0.78 ha), including the L. pulmonaria host tree, was higher than in the more distant zones, despite the radius increasing the size of the next zone R = 51–100 m (2.355 ha) and R = 101–150 m (3.925 ha) (see Table 2). The share of the number of all species found in the R = 50 m zone in the total lichen diversity, reaching an average of 68%, could be considered by many as a satisfactory level, fulfilling the function of an umbrella species in an efficient nature protection system. Nevertheless, the extension of the protection zone to the surface area it had before 2014 shows that it would protect almost 90% of the epiphytic lichen biota growing under the umbrella of L. pulmonaria. It is obvious that, up to a certain limit, the number of species increases significantly with the increase in the studied area (Potenza et al., 2022). In a relatively homogenous environment, where communities are devoid of prominent dominants (as indicated by the high Pielou homogeneity index (J = ±0.8), equalized in successive zones), this increase does not, however, imply statistically significant differences in the number of species between successive zones, as in the cases we studied. The quantitative analysis of species alone does not seem to be sufficient to fully assess the quality of the environment and the role of L. pulmonaria in the protection of key forest habitats for stenobiont lichens due to the considerable ecological plasticity of many forest species and, at the same time, the availability of many ecological niches that can be inhabited by a rich set of epiphytes in a homogenous macro-habitat wide zone (R = 150 m, i.e. ±7 ha).
The results of relative biological unification expressed by similar values of Shannon-Wiener (H) and Pielou (J) indices can be considered in two ways. On the one hand, they testify to the representative ecological nature of the umbrella protection zone. On the other hand, they prove that the areas outside the current protection zone are equally valuable in terms of species diversity and should also be treated as worth conserving. The assessment of umbrella protection looks different in terms of the possibility of protecting species that prefer primeval forests and highly endangered species. During the research, a total of 25 species from the first group and 30 red-listed species related to CR and EN categories (some of them represent both groups) were found (Figure 5, Figure 6). In both cases, the R = 50 m zone protected from 58% to as much as 72% of species, but increasing the zone to 100 m allows protection from 9% to 37% more valuable species. It should be noted that these species were relatively rare. Some of them were found only at single sites. Taking into account the results of the methodological work by Vondrák et al. (2016) in forests of a relict, primeval nature, it should be expected that, especially in the pool of endangered species, the share of rare microlichens could be even higher. The most numerous species was Loxospora elatina. Its presence was recorded 48 times, which translates into only 12% of all examined trees. It is also worth noting that few valuable species occurred in zones up to 50 m. Most of them were also present in further zones or only in them. This was the case among others, in the case of critically endangered species (CR category), of which only two out of six found occurred in the immediate vicinity of the L. pulmonaria host tree. Except for Menegazzia terebrata, each critically endangered species has only been recorded once.
Analyzing the lichen biota of valuable species, it can be concluded that the protection zone R ≤ 50 m is insufficient for their full protection. These species also occur further away from the site of L. pulmonaria, and their small number makes it necessary to protect each thallus. Enlarging the zone within a stable forest habitat increases the dispersion success of lichens. L. pulmonaria, as an umbrella, can play a very important role here. The protection of lichen species alone may be insufficient; in many cases it is also necessary to protect a habitat appropriate for the species in which dispersion has a chance of success. Optimal protection of lichens also requires knowledge of the biology of very rare and endangered species populations, which will help to develop an appropriate strategy for their protection (Scheidegger & Werth, 2009).
In the case of primeval forest indicators and endangered species, clear differences in the number of species were recorded after the extension of the protection zone to R = 100 m. In Gorce Mts, it could result in an increase in the share of protected species by 6% and 9%, respectively. The situation is different in the Bieszczady Mts, where this increases by 28% and 37%, respectively, which is as much as 1/3 of all species in this region. A much higher percentage of species found at a greater distance from the host-tree of L. pulmonaria in the Bieszczady Mts may nevertheless prove a less homogenous ecosystem manifested by a greater variety of habitats; perhaps resulting from different use of these forests?
. Recommendations for forest management resulting from nature protection reasons
Treating the zone protection of Lobaria pulmonaria as a tool for protecting forest biodiversity and creating refugia for valuable and endangered representatives of the Carpathian and Polish lichen biota.
Where possible, applying the legally required protection of forest lichen species in zones with a radius of not less than 100 m.
Exclusion from planned use of forest parts related to area division in places where species requiring zone protection are more abundant.
Inventory of species requiring zone protection at the stage of preparing forest management plans, along with planning the area scope of protection zones.
. Supplementary material
The following supplementary material is available for this article:
Table S1. Results of the analysis of Dunn’s multiple (two-tailed) comparisons between numbers of lichen species found at each sampled site located in the Polish Carpathians. Significant differences at p < 0.05 level have been highlighted in red.


