. Introduction
Representatives of the family Celastraceae are distributed in tropical and subtropical regions, such as Northern Africa, South America, and Eastern Asia (Simmons et al., 2008). This family comprises 96 genera and approximately 1,350 species (Christenhusz & Byng, 2016); besides, it is represented by three genera in Brazil: Maytenus Juss., Austroplenckia Lund., and Franhofera Mart. (Carvalho-Okano, 1992). The genus Austroplenckia is represented by a single species, Austroplenckia populnea (Reissek ex Mart.) Lundell, which is a homotypic synonym of Plenckia populnea Reissek (SiBBr, 2020). A. populnea is popularly known as “marmelinho-do-campo” and can be naturally found in the Brazilian Cerrado biome (Andrade et al., 2007).
Several studies have been carried out to identify the secondary compounds of different plant species belonging to this family and to better understand their pharmacological properties (Caneschi et al., 2015; Espindola et al., 2018; Mokoka et al., 2013; Nikolova et al., 2021; Nizer et al., 2021; Rodrigues et al., 2015; Santos et al., 2013; Spivey et al., 2002). Extracts deriving from Celastraceae plant parts have been used as insect repellents and insecticides in traditional agriculture in South America and China (Deepa & Narmatha Bai, 2010). Several sesquiterpenes stand out among bioactive compounds found in this family of plants, since they have the potential to be used for the aforementioned purposes (Avilla et al., 2000; Deepa & Narmatha Bai, 2010; Li et al., 1997; Martins et al., 2021; Spivey et al., 2002).
The fact that A. populnea belongs to the Celastraceae group explains the interest in pharmacological studies focused on investigating its crude or isolated and identified bioactive extracts. Among them, there are studies conducted by Andrade et al. (2007, 2008), Arini et al. (2019), Caneschi et al. (2015), Miranda et al. (2009), and Seito et al. (2002). Most of them highlight several terpenes found in different plant parts, e.g. leaves. Miranda et al. (2009) have identified the following pentacyclic triterpenes (TTPCs) in the leaves, branches, bark, and roots of this plant species: friedelin, 3β-friedelinol, 28-hydroxy-friedelan-3-one (canophyllol), populnonic acid, catononic acid, epicatonic acid, pristimerine, abruslactone A, α-amyrin, methyl populnonate, methyl catotonate, and methyl epicatonate.
In addition to terpenoids, Caneschi et al. (2015) has emphasized the incidence of one flavonoid and one undecanamide in this plant samara extracts. The aforementioned author has drawn attention to the bioactive potential of this plant species and to the need of accurate exploration of its phytochemical components as well as other possibilities of using it as a drug or for other purposes.
Fall armyworm (Spodoptera frugiperda) (Lepidoptera: Noctuidae) is a polyphagous species that attacks different economically important crops in different countries. It is the main pest attacking maize crops, since it causes damage from the seedling to the ear formation stage (Gilal et al., 2020). As reported by Cruz (1995), in Brazil, reductions in corn yield due to the attack of this pest range from 15 to 34%. The percentage of damage depends on the development phase of the plant in which the attack occurs. Currently, the main methods used for its control involve the use of organosynthetic insecticides and transgenic plants. However, routine spraying with insecticides from the same group of action, associated with overlapping or successive crops that include host plants, exposes the populations of S. frugiperda to high selection pressure by insecticides and Bacillus thuringiensis (Bt) proteins, which endanger the available control tactics (Bezerra et al., 2019). Therefore, alternative methods that are in line with integrated management programs for this pest, such as the use of insecticidal plants, should be studied.
To the best of our knowledge, despite the pharmacological interest in A. populnea, the literature still lacks studies about the potential insecticidal properties of extracts deriving from the leaves and from other organs of this species. Thus, the aim of the current study was to investigate the toxicity of crude and fractionated extracts from A. populnea leaves in a S. frugiperda population.
. Material and methods
Experimental site
Plant extract obtainment procedures were carried out at Henrique Santillo Chemistry Laboratory, State University of Goiás, Anápolis County, Goiás, Brazil. Biological tests were conducted at the Entomology Laboratory of Universidade Estadual de Goiás, Ipameri County, GO, Brazil, in a climatized room, at the mean temperature of 25 °C ± 2 °C, mean relative humidity of 70% ± 10%, and 12-hour photoperiod.
Maize growing to feed S. frugiperda larvae
As recommended by Bezerra et al. (2019), the substrate used to grow maize in 8-litre pots comprised a mix of red yellow latosoil sand and cattle manure at the ratio 2:1:1. Three non-transgenic hybrid seeds (RK 9014) were sown in soil at a depth of 2.5 cm per pot. Plants grown from these seeds were kept in a greenhouse and irrigated daily. Base fertilization with N–P–K (08–20–20) and top dressing with granulated urea were carried out. To avoid contaminations, no insecticide or fungicide applications were performed throughout the crop development cycle.
Austroplenckia populnea leaf extract preparation, phytochemical prospection, and isolation of organic compounds
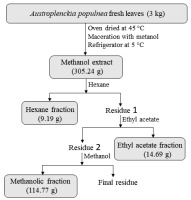
Plant extracts were obtained from leaves of adult A. populnea plants (3.0 kg), collected in the native Cerrado region (16° 21′ S; 48° 55′ W), nearby the Anápolis municipality, Goiás, Brazil. The leaves were dried in an oven at 45 °C for 48 hours, crushed in a knife mill, and immersed in pure methanol in three 2.0-litre Erlenmeyer flasks. Crude extract was obtained through cold maceration of this material, which was then filtered and concentrated in a rotary evaporator. Crude extract fractionation was carried out in solvents at ascending polarity order (hexane, ethyl acetate. and methanol again). This process was carried out through vacuum filtration after microcrystalline cellulose D adjuvant incorporation to the methanol extract. The produced extract had viscous and dark appearance.
The concentrations of the crude methanolic extract and the hexanic, ethyl acetate, and methanolic sub-fractions used in the assays were 0.01, 0.1, 0.5, 1.0, and 5.0% (w/v).
These concentrations were established based on preliminary tests, which were assumed to fit a representative dose-response curve due to the amount of extract available. Preliminary tests were also used to select the solvent that best dissolved the crude and fractioned extracts, i.e., deionized water + acetone at 1:1 ratio, and that did not have any repellency effect after its evaporation from the surface of the maize leaves provided as food for larvae. This water + acetone mix was used as a control treatment.
The preliminary phytochemical prospection of A. populnea leaves was carried out to identify the following compounds: phenols (simple phenols, flavonoids, anthraquinones, coumarins, and tannins), nitrogen compounds (alkaloids), and terpenes (cardioactive heterosides and saponins). The adapted methodology applied to phytochemical prospecting can be found in Costa (2001) and Matos (2009).
Since the chemical compounds of A. populnea are well-known, the current study has only recorded chemical compounds (isolated or not) identified in its leaves, with emphasis on the anti-feeding effects of the extracts on the investigated S. frugiperda population.
Toxicity of A. populnea leaf extracts towards S. frugiperda
The newly emerged larvae used in our experiment came from females reared on artificial diet, based on the methodology proposed by Greene et al. (1976). The natural diet used in the current bioassays comprised young leaves of maize plants. As soon as they emerged from the eggs, the larvae were isolated in 250 mL plastic cups (n = 20) filled with part of the conventional maize plant (RK 9014) and treated with different A. populnea extracts. The treatment consisted in dipping the leaves in different extracts, waiting for the solution to fully dry in the shade, and providing the leaves to the neonate larvae. The bottom of the plastic container was lined with filter paper and lightly moistened with distilled water. The larvae were allowed to feed on the treated leaves for 36 hours. The consumption of fresh leaves by single larvae was determined by weighing at 10 days on an analytical balance accurate to 0.001 grams. After the period of feeding on maize leaves treated with the extracts was over, the larvae received only conventional untreated maize ad libitum. The food provided was changed on a daily basis, their plastic containers were cleaned to eliminate faeces and exuvia, and the filter paper was changed and moistened with water. This procedure was maintained until pupal formation.
Pupae were weighed and sexed 24 hours after their full formation and taken back to their respective containers to enable monitoring and recording other biological variables. The number of pupae used in each treatment corresponded to the number of pupae remaining from the previous treatment with the extracts. After adult insects emerged, they were kept in the same pots as in the larval stage; however, they were not provided with any food other than cotton soaked in distilled water on a daily basis.
The following biological variables were measured: duration of larval and pupal stages, larval and pupal mortality, larval weight at 10 days, pupal weight within 24 hours after pupal formation, adult longevity, and number of adult individuals with morphological abnormalities (the final shape of the wings without deformable areas) visible to the naked eye. The study followed a completely randomized experimental design with 21 treatments.
Statistical analyses
Data on food intake per larvae, larval and pupal mortality, pupal and larval weight, duration of larval and pupal stages, larval and pupal weight at 10 days, 24-hour pupal weight, and adult longevity were subjected to analysis of variance. The current data did not meet normality and homoscedasticity assumptions. Thus, the variables were ranked for multiple comparisons between treatments, based on the Scott-Knott or Chi-square (χ 2) tests. The specific extract dose/larval and pupal mortality ratio was checked through the binomial regression model at P < 0.05. All analyses were carried out in R software, version 3.6.2 (R Core Team, 2021).
. Results
Crude methanolic extract (CME) and its fractions were obtained from the dried leaves of A. populnea (Figure 1). The phytochemical prospection of the A. populnea leaves showed the following groups of secondary metabolites: flavonoids, steroids, triterpenes, saponins, and tannins; in turn, no alkaloids and coumarins were detected. These compounds have been previously isolated from species of the family Celastraceae (Camargo, 2022; Spivey et al., 2002; Veloso et al., 2017).
The Austroplenckia populnea leaf extracts applied to maize leaves and provided as food to S. frugiperda promoted a significant reduction in food consumption by larvae (Table 1). The variants with the A. populnea extract [CME, hexane fraction (HF), and methanol fraction (MF)] differed from the control treatment. The CME, HF, and MF treatments (except for the concentration of 0.5%) decreased the body weight of S. frugiperda larvae at 10 days. On the other hand, the treatments with the highest extract concentrations (except for methanol at 1%) did not significantly reduce the weight of formed pupae (Table 1).
Table 1
Food intake, mean individual larval weight 10 days after feeding on treated leaves, pupal weight 24 hours after pupal formation, larval and pupal mortality rates recorded for different treatments (maize leaves treated with different Austroplenckia populnea extracts at different concentrations provided to third instar Spodoptera frugiperda larvae). Maize leaves treated with extracts remained available for consumption by larvae for 24 hours.
[i] Means and respective standard deviations followed by the same letter in the column do not differ statistically from each other in the Sckott-Knott test at 5% significance. Larval and pupal mortality data from each extract treatment were compared to the control treatment (Chi-squared at P < 0.05). There were no significant differences in pupal mortality between the treatments with the extracts and the control (Chi-square at P < 0.05). ns = not significant. Control = untreated larvae. % w/v = % weight per volume. CME = Crude methanolic extract. HF = Hexanic fraction. EAF = Ethyl acetate fraction. MF = Methanolic fraction.
There was a significant difference in S. frugiperda larval mortality among the treatments. Hexane extracts from A. populnea leaves stood out. Several extract concentrations used in the other treatments (CME, EAF, and MF), including the highest ones (1 and 5%), contributed to larval mortality rates similar to that of the control. The logistic regression results did not show significant association between the S. frugiperda larval mortality rate and the concentration of each A. populnea leaf extract. No treatment with the leaf extracts deriving from the tested plant made the pupal mortality rate significantly different from that of the control treatment (χ 2 = 16.468; P = 0.687).
The higher consumption of leaves treated with EAF resulted in a reduction in the duration of the larval stage (Table 2). However, with the exception of the same extract, at a concentration of 0.01%, there was no significant change in the duration of the pupal stage (Sckott-Knott test at P < 0.05). Also, none of the tested extracts promoted significant changes in the adult longevity and sex ratio of the insect population tested (Chi-Square at P < 0.05).
Table 2
Variables used to measure the anti-feeding effect of Spodoptera frugiperda individuals fed on maize leaves treated with different Austroplenckia populnea leaf extract fractions and concentrations.
[i] Means with respective standard deviations followed by the same lowercase letter in the column do not differ statistically in the Sckott-Knott test at P < 0.05. Sex ratio data from each extract treatment were compared to the control treatment (Chi-squared at P < 0.05). Control = untreated larvae. % w/v = % weight per volume. CME = Crude methanolic extract. HF = Hexanic fraction. EAF = Ethyl acetate fraction. MF = Methanolic fraction.
. Discussion
The pharmacological properties and phytochemical characterization of A. populnea have been relatively well studied in Brazil (see Andrade et al., 2007; Andrade et al., 2008; Caneschi et al., 2015; Miranda et al., 2009; Seito et al., 2002).
Secondary metabolites, i.e. flavonoids, steroids, triterpenes, saponins, and tannins identified in the extracts of this plant in our study, have already been mentioned as potential toxic agents against insects. For example, certain flavonoids, such as ermanin and rutin, act as antifeedant agents (Echeverri et al., 1991; Silva et al., 2016) and insect-growth inhibitors (Simmonds, 2003), likely because they interfere in neural mechanisms whose toxic action is influenced by the chemical structure of each specific compound. The biological activities of terpenes are yet to be fully investigated. As shown by Breitmaier (2006), several plant species produce volatile terpenes to attract specific insects for pollination. They also produce lesser volatile terpenes that have an anti-feeding effect, which is the likely function of the mix of betulinic acid, α-amyrin, and β-amyrin found in the herein investigated plant species. Cardioactive glycosides are other terpenoids found in A. populnea; they are well-known drugs capable of influencing vertebrate’s cardiac muscles (Taiz et al., 2014); however, they have not yet been investigated in insects. Saponins (glycosides) work as defence compounds. Tian et al. (2020) reported the toxicity of triterpene saponins in Plutella xilostella (L.) larvae. Tannins found in A. populnea leaves act as a protective barrier against herbivorous insects (Kvedaras & Keeping, 2007; Massey et al., 2006).
All treatments, except for the EAF variant, had an antixenosis effect on neonate S. frugiperda larvae. The reduced intake of maize leaves treated with A. populnea leaf extracts by insect larvae is an important feature to be taken into consideration at the time of selection of repellent substances. However, such repellency may have influenced the response of the antibiosis effect variables investigated in the current study (larval and pupal weight, larval and pupal mortality rate, sex ratio, and duration of insects’ juvenile and adult stages). Such a reduction in leaf intake, in combination with the intake of toxic compounds deriving from the extract, influenced larval and pupal mortality rates.
The type of ingested extract affected the larval mortality rate. HF deriving from A. populnea leaves resulted in a higher larval mortality rate at all investigated extract concentrations. Bezerra et al. (2019) and Sousa Neto et al. (2018) have also highlighted larval mortality rates recorded for hexane extracts deriving from leaves of Brazilian Cerrado native plants, such as Andira paniculata Benth. and Machaerium opacum Vogel with insecticidal potential against Helicoverpa armigera Hübner and S. frugiperda, respectively. Other extracts resulted in a significant larval mortality rate, although it was not as significant as the mortality rate recorded for extracts deriving from the hexane solvent. Values recorded for lethal insecticide concentrations extrapolated for a given population are often expressed based on 50% of treated organisms (LC50). However, the data in the current study did not provide a significant regression model between the extract concentration and the larval/pupal mortality rates to estimate such a reference. This dose/mortality non-dependence is also common in similar studies (Bezerra et al., 2019; Sousa Neto et al., 2018). Due to the extract intake by the neonate larvae, the pupal mortality rate was low, and none of the adopted treatments differed from the control. Adult individuals did not show perceptible anomalies.
Some A. populnea extract fractions significantly changed the duration of insects’ life cycle stages in comparison to the control. Overall, plant extracts used at lower concentrations (0.01 and 0.1%), regardless of the solvent, reduced the cycles (in days) of larval, pupal, and adult stages. It is worth emphasizing that the extract concentration to which the feeding larvae were exposed is not always the one that actually reaches the cells, sites, and membranes where reactions accounting for their toxic effect take place. As suggested by Sendi and Ebadollahi (2013), the biological activity of a given substance may depend on its interaction with other substances in the environment (in the present case, insects’ organism), which, in some cases, are not even toxic. In most cases, this makes it impossible to define a single substance accounting for a certain toxic effect.
Spodoptera frugiperda is an extremely relevant maize crop pest that has been historically controlled with organosynthetic insecticides. Therefore, alternative natural insecticide compounds must be constantly investigated by programs focused on the integrated management of this pest. Among the results presented in the current study, it is worth emphasizing the larvicidal effect of extracts (mainly HF) from A. populnea leaves, as it may open possibilities to use its secondary metabolites to control this and similar pests.