. Introduction
Berries are becoming increasingly popular for being a source of bioactive compounds with beneficial effects on human health (Gündeşli et al., 2019; Okatan, 2018). Strawberry, raspberry, blackberry, currant, gooseberry, blueberry, rosehip, and jackal plum are widely consumed and have significant commercial value; while less important species, such as buffaloberry, serviceberry, cloudberry, and farkleberry, occur in forested areas or are typically used as ornamental plants (Okatan, 2020). In view of the potential of the berries, farmers must learn more about propagation and cultivation techniques for the unrecognized or wild species, as they may lead to crop diversification.
In Peru, 13 wild species of Vaccinium have been reported (Mostacero et al., 2015). However, their potential cultivation has received little research, and their use is limited to fresh fruit consumption, as they are present only in natural populations. The growing demand for land use for urban growth and the expansion of agricultural areas poses a risk to germplasm conservation (Ligarreto et al., 2011).
Despite the potential of wild species, efforts to establish propagation methods to expand the cultivation of new blueberry species are limited. Blueberry can be propagated by sexual and asexual pathways; the latter pathway produces homogeneous descendants, which ensures the transfer of genetic potential and provides materials to establish commercial crops in a short period (Braha & Rama, 2018). A simple alternative is propagation using cuttings, wherein the development of adventitious roots is induced through the application of exogenous hormones. Several studies have suggested that the rhizogenic capacity of blueberry cuttings is influenced by both the cultivar and the type of growth regulator (Castrillón et al., 2008; Peña et al., 2012).
Therefore, the objective of the present study was to evaluate the effects of different concentrations of indole-3-butyric acid (IBA) on the rooting of cuttings of five wild blueberry accessions (Vaccinium spp.). The results are intended to facilitate the regeneration of new plants and their subsequent commercial use and to contribute to sustainable agrobiodiversity management.
. Material and Methods
Identification and Selection of Mother Plants
The propagation material was identified from natural populations located in the province of Chachapoyas, Peru. Five accessions of wild blueberries were selected (Table 1) after rejecting plants with visible signs of phytosanitary problems and nutritional deficiency.
Table 1
Geographic locations of five accessions of wild blueberries.
| Blueberry accession* | Geographic coordinates | Altitude | District | ||
|---|---|---|---|---|---|
| South latitude | West longitude | (m a.s.l.) | |||
| HCHA-286 | 06°28′53.4″ | 77°47′39.5″ | 2,855 | La Jalca | |
| HCHA-271 | 06°12′59.5″ | 77°40′43.5″ | 2,462 | Molinopampa | |
| HCHA-262 | 06°43′22″ | 77°55′42″ | 2,942 | Leymebamba | |
| HCHA-283 | 06°10′36″ | 77°47′11″ | 2,509 | Chachapoyas | |
| HCHA-290 | 06°07′50.8″ | 77°52′34.4″ | 2,680 | Huancas | |
Collection of Plant Material
To obtain cuttings of similar chronological ages, the mother plant was pruned. Subsequently, phytosanitary controls were established with carbendazim at 1.50 mL L−1 (Caberxim 500 SC; Nanjing Rhonquim, China), and shoot development was stimulated with a trihormonal inducer at 0.5 mL L−1 (Big-Hor Plus; Grupo Andina, Peru); both compounds were applied every 15 days for 8 months.
Semihardwood shoots (diameter greater than 0.5 cm) were then collected from the middle third of the plant. The plant material was disinfected by immersion in a 2 g L−1 mancozeb solution (Mancozeb 80 WP; Limin Chemical, China) and benomyl at 0.5 mg L−1 (Benomyl 50 WP; Taicang Pesticide Factory, China) for 5 min.
Establishment of the Experiment
The experiment was carried out in a low tunnel greenhouse (6 m long, 3 m wide, and 1.50 m high) under the shade of 70% black Raschel mesh. A nebulization system was installed to maintain temperatures between 20 °C and 25 °C and a relative humidity above 80%.
The shoots were cut into 8-cm segments (with a 45 ° bevel cut at the base), and four leaves were left with 50% of their leaf area. For each cutting, the basal end (0.5 cm) was immersed for 1 min in ultrapure water (control) or in one of several different concentrations of IBA (1,000, 2,000, or 3,000 mg L−1) and then seeded in germinating jars containing a sterile peat-based substrate (pH: 4.5; organic matter: 7.38%) and perlite at a ratio of 4:1.
Experimental Design and Data Analysis
A completely randomized experimental design with a factorial arrangement (five wild accessions × four concentrations of IBA) with 20 repetitions per treatment was used. After 45 days, the rooting and callus percentages and the numbers and lengths (mm) of roots and shoots were evaluated.
The normality of the data was verified using general and mixed linear models, followed by square root transformation for shoot length. The data were subjected to analysis of variance, and the means of the treatments were statistically compared using the Di Rienzo–Guzmán–Casanoves (DGC) test (Di Rienzo et al., 2002) at a 5% probability of error. Analyses were performed using the statistical package InfoStat version 2017. Likewise, polynomial regression analysis (p ≤ 0.05) was performed on the IBA concentration. The percentages of rooted cuttings and cuttings that formed calluses were obtained using Microsoft Excel.
. Results
Rooting and Callus Formation Percentages
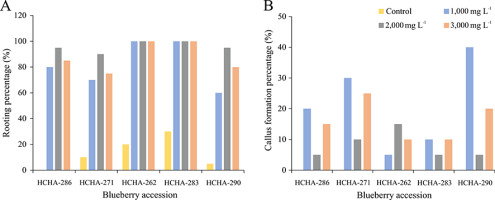
Wild blueberry cuttings responded favorably to IBA application, reaching rooting percentages higher than 60%. The highest number of rooted cuttings was recorded in the treatment of 2,000 mg L−1 IBA (Figure 1A). In particular, accessions HCHA-262 and HCHA-283 achieved 100% rooting and showed the highest root development among the accessions under all three IBA concentrations (Figure 2). Callus formation was observed only in cuttings treated with IBA (Figure 1B).
Numbers and Lengths of Roots and Shoots
The data analysis revealed that both IBA concentration and accession type had a significant influence (p ≤ 0.05) on the number and length of roots and shoots (Table 2). Regression analysis of the number and length of roots in all the evaluated accessions showed that the highest means were obtained when 2,000 mg L−1 IBA was used and that higher IBA concentrations impaired root formation and elongation (Figure 3A,B). Furthermore, in all the accessions analyzed (except HCHA-262), the shoot number and length were negatively affected by IBA concentrations higher than 2,000 mg L−1 (Figure 3C,D).
Table 2
Effects of indole-3-butyric acid (IBA) on the numbers and lengths of roots and shoots (± standard deviation; SD) of five wild blueberry accessions.
Figure 3
Polynomial regression graphs of the (A) number of roots, (B) length of roots, (C) number of shoots and (D) length of shoots in wild blueberry accessions treated with increasing concentrations of indole-3-butyric acid (IBA).
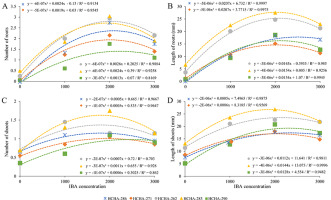
Accessions HCHA-286, HCHA-262, and HCHA-283 treated with 2,000 mg L−1 IBA presented higher number of roots than the other accessions (although they were not always the largest), with an average range between 2.75 and 3.05 roots (Table 2). The highest average values for the root number (1.75) and shoot length (26.84 mm) were observed in the HCHA-283 accession using IBA at 2000 mg L−1 (Table 2).
. Discussion
Using cuttings for propagation can be an effective strategy to bring wild berries closer to farmers and initiate their cultivation. However, some studies have reported that blueberries have a low rooting rate, which limits their propagation (Braha & Rama, 2018; Koyama et al., 2019). In the current study, the rooting percentages of all five accessions were greater than 90% after treatment with 2,000 mg L−1 IBA. Additionally, with this treatment, the accessions achieved the greatest root and shoot formation and elongation. Similar results were reported in studies of the blueberries ‘Bluebelle,’ ‘Bluegem,’ and ‘Powderblue’ (Marangon & Biasi, 2013) and mora ‘Xavante’ (Hussain et al., 2014), where the best rooting response was observed at this IBA concentration.
The results showed that the rhizogenic capacity and shoot development of the cuttings varied owing to the influences of the germplasm and IBA concentration. IBA at high concentrations has been reported to potentially have inhibitory effects on vegetative propagation (Koyama et al., 2019), although such effects may vary because of factors such as genetic variation among species or cultivars (An et al., 2018) and variations in the application method (liquid or powder) (Peña et al., 2012) and type of explant (apical, middle, or basal) (An et al., 2018). Therefore, for the propagation of wild species for which plant materials may be difficult to obtain, it is important to determine the ideal IBA dose for producing the maximum number of plants.
Interestingly, the cuttings under the control treatment developed roots and shoots, despite not receiving IBA. Nascimento et al. (2011) found similar results, reporting that the rooting percentage in cuttings that had not been treated with phytohormones was greater than 50%. These results may be related to the presence of leaves on the cuttings, as the leaves are an endogenous source of auxins (Castrillón et al., 2008; Marangon & Biasi, 2013). Therefore, verifying the physiology, nutrition, and health of the mother plant is important (An et al., 2018; Pacholczak & Nowakowska, 2020) because these factors are related to the availability of reserve nutrients and the endogenous levels of hormones at various stages of development (Braha & Rama, 2018). These observations indicate that plant preparation can be a determining factor for the success of clonal multiplication.
. Conclusions
The use of IBA was effective for the propagation of wild blueberries through cuttings. In the evaluated accessions, the number and length of roots and shoots showed the best results with the application of 2,000 mg L−1 IBA. The five accessions showed differences in rooting capacity, indicating that they require different conditions for their propagation.