. Introduction
The use of traditional medicinal plants for the purposes of pain relief and disease treatment has been growing in popularity worldwide (Ghaleb Dailah, 2022; Hemeg et al., 2020), which may be due to their high content of phenolic compounds, such as flavonoids and anthocyanin (Al-Otibi et al., 2021). Several studies have demonstrated that various medicinal plants contain antimicrobial compounds that can effectively inhibit the growth of various microorganisms. These compounds may possess higher potency than the currently available antibiotics (Almabruk, Dinh & Philmus, 2018; Reker et al., 2014; Ruddaraju, Pammi, Guntuku, Padavala & Kolapalli, 2020; Shankar, Rangarajan, Sarada & Kumar, 2010; Singh et al., 2023). The Artemisia genus (f. Asteraceae), which comprises 500 species of small bitter herbs and shrubs distributed typically across North America, Asia, and Europe (Abdullah et al., 2021; Barreda et al., 2015; Silva, 2004). The majority of the species of this genus have gained significance in the world due to their antimicrobial, antioxidant, insecticidal, and antimalarial activities and the ability of some of them to be used for cancer treatment (Abad, Bedoya, Apaza & Bermejo, 2012; Chhetri, Al-Sokari, Setzer & Ali, n.d.; Kalemba, Kusewicz & Świąder, 2002; Petretto et al., 2013; Qanash et al., 2023; Silva, 2004). The Artemisia genus is represented by six species in Saudi Arabia (A. scoparia, A. monosperma, A. sieberi, A. absinthium, A. abyssinica, and A. judaica) (Guetat, Al-Ghamdi & Osman, 2017; H. A. Mohammed, 2022; Qureshi et al., 1990). Among the most important Artemisia species is Artemisia abyssinica, which is known as “boitheran” in Saudi Arabia and Yemen (Chhetri, Awadh Ali & Setzer, 2015; S. Mohammed, Dekabo & Hailu, 2022). Plants produce a wide range of bioactive substances, such as essential oils that can have various effects on living organisms. The composition and properties of these essential oils can differ, depending on the plant species, the geographic location, and the environmental conditions in which they are cultivated. Researchers have analyzed the essential oils of this plant to determine the diversity of its chemical constituents across different regions and settings (Chhetri et al., n.d.; S. Mohammed et al., 2022; Pandey & Singh, 2017). To the best of our knowledge, no research to date has examined the extraction of the essential oils of Artemisia abyssinica that has been grown in the Al-Baha region, particularly in Almukwah province, which is characterized by temperate weather in winter, making it an ideal location for growing this species of plants. The aim of this study is to explore the potential applications of the essential oil of A. abyssinica with different activities in medicine.
. Material and methods
Plant materials
The aerial parts of Artemisia abyssinica Sch.Bip. ex Oliv. & Hiern were collected from a farm in Al-AlMukwah, Saudi Arabia, in late December 2022. Dr. Nageeb A. Al-Sagheer from the Biology Department at the Faculty of Sciences, Al-Baha University, Saudi Arabia, verified the identity of the plants. The plant samples (without number) were stored in the herbarium of the Department of Biology at this university.
Extraction of essential oil
The fresh aboveground parts of A. abyssinica (200 g) were washed with distilled water, dried, chopped into small pieces, and then used to distill the essential oil using Clevenger apparatus for 4 h after boiling. The obtained oil was dried using sodium sulfate (anhydrous) and stored in a closed container in a fridge at 4 °C for later analysis (Al-Ghamdi, Fadlelmula, Abdalla & Zabin, 2021).
GC/MS analysis of essential oil
The GC/MS analysis was conducted using a Thermo Scientific Trace GC Ultra/ISQ Single Quadrupole MS instrument equipped with a TG-5MS fused silica capillary column (30m long, 0.251mm in diameter, and with a 0.1 mm film thickness). For the GC/MS analysis, an electron ionization apparatus with an ionization energy of 70 eV was employed. Helium was utilized as the carrier gas, maintaining a consistent flow rate of 1 mL/min. The temperature of the injector and the MS transfer line was fixed at 280 °C. The oven was set to an initial temperature of 40 °C, which was maintained for 3 minutes. Subsequently, the temperature increased at a rate of 5 °C per minute until it reached a final temperature of 280 °C. This final temperature was then maintained for 5 minutes. The identification of all of the oil components was accomplished by employing a percentage relative peak area. The compounds were provisionally detected by comparing their respective retention time and mass spectra with the data published in the NIST and WILLY library for the GC/MS system.
Antimicrobial assay
To test the in vitro antimicrobial activity of the oil, we used the agar well diffusion method to test the level of protection against five bacteria and three fungi. The bacteria were two Gram-positive (Staphylococcus aureus (ATCC:13565) and Streptococcus mutans (ATCC:25175) and three Gram-negative (Escherichia coli (ATCC:10536), Pseudomonas aeruginosa (ATCC:27853), and Klebsiella pneumoniae (ATCC:10031)). The fungi were Candida albicans (ATCC 10231), A. brasiliensis (ATCC 16404), and Aspergillus ochraceus (ATCC:22947). All the tested strains and the control were delivered from the microanalyses center at Cairo University, Faculty of Sciences, Egypt. Nutrient agar and Sabouraud dextrose agar were used for the bacterial and fungal growth, respectively. We compared the inhibition zones produced by the oil with those of Ampicillin, Gentamicin, and Nystatin, as the conventional antimicrobial agents. Also, a negative control was prepared by dissolving the oil in dimethyl sulfoxide (DMSO). The oil was tested at the 15 mg/ml concentration against all of the microorganisms.
Plant materials
The following steps were performed to test the antibacterial activity of the compound. Firstly, each of the Petri plates was sterilized and filled with 20–25 ml of sterilized medium, then allowed to solidify at room temperature before proceeding to the next step. Next, the microbial suspension was adjusted with a sterile saline solution to obtain a concentration equivalent to the McFarland 0.5 standard solution (1.5× 105 CFU mL-1), which corresponds to an optical density (OD) of 0.13 at 625 nm, as measured by a spectrophotometer. The other procedures as reported in literature (Mackie, 1989).
The swab was then placed inside the closed lid after 15 minutes of drying. A sterile borer was used to make 6 mm wide wells in the solidified material. 100 μL of the solution with the tested compound was added to each well using a micropipette. Finally, the plates were incubated at 37 °C for 24 hours to evaluate possible antibacterial activity. The experiment was repeated three times, and the inhibition zones were measured with a millimeter scale (Mackie, 1989).
Determination of MIC
We isolated 3–5 colonies of each strain from the fresh agar plate and transferred these to a tube containing 3–4 ml of sterile broth medium. We adjusted the bacterial suspension to match or exceed the turbidity of the McFarland Standard 0.5, and then 1 mg of the tested compound, which is an antimicrobial agent, was dissolved in 1 ml DMSO. We performed two-fold serial dilution of this solution using a broth medium. We added a specific amount of the bacterial inoculum to each tube and incubated them at 37°C for 16–20 hours. For the fungal inoculum, the incubation time was 24–48 hours. The MIC is the lowest amount of an antimicrobial agent that can inhibit the visible growth of the test isolate, without requiring any magnification tools (Chudáčková et al., 2010; Wiegand, Hilpert & Hancock, 2008).
Antitumor activity study
Cell culture and treatment: hepatocellular carcinoma (Hepg2)
We cultured a cell line from the National Cancer Institute as a “monolayer culture” in RPMI medium supplemented with 10% fetal bovine serum (FBS) and 2% penicillin/streptomycin (Pen/Strep). We incubated the cells at 37°C in a humidified atmosphere with 5% CO2 using a water jacketed incubator from Thermo Fisher Scientific, USA. This mimicked the human body conditions and facilitated cell growth and differentiation. The incubation duration depended on the cell type and the experimental plan. To maintain the cells in the exponential growth phase, we performed frequent sub-culturing. We worked in a sterile environment using a Microflow Laminar flow cabinet (MDH limited, Hampshire SP105AA, UK) to remove any particles. We divided the cells into two groups: a control group and a treatment group, which received various concentrations of the drug (12.5, 25, 50, and 100 μg/mL).
Cell proliferation assay
To assess cell viability, we used the used the MTT assay as follows. We incubated the cells for 24 hours and then added 10 μl of the MTT solution (0.5 mg/ml) to each well. The incubation period was extended for a further 4 hours. Next, we added 100 μl of a solubilization solution to dissolve the formazan crystals formed by the viable cells. We measured the absorbance of the samples at 570 nm using a microplate reader. We calculated the cell viability percentage using the following formula:
Cell viability (%) = [ODS/ ODC] × 100.
Where ODS is the mean optical density of the sample and ODC is the mean optical density of the control (Ferrari, Fornasiero & Isetta, 1990).
Molecular docking
We applied density functional theory (DFT) with the B3LYP functional and the 6–311G (d) basis set to optimize the molecular structures of the isolated oil. We performed molecular docking studies to examine the interactions between the essential oil and the active site of Staphylococcus aureus dihydrofolate reductase (DHFR) using PDB code 3SR5. We prepared the 3D crystal structure of DHFR using Schrödinger’s Protein Preparation Wizard “Maestro Schrödinger”, by adding polar H-atoms, removing nonpolar H-atoms, and assigning charges. We used the Glide tool to perform the molecular docking, using a grid map of 25×25×25 centered on the binding pocket (Jalali Heravi & Sereshti, 2007).
Statistical analysis
To compare the variation among groups of the same microbial species, we applied a one-way ANOVA to the data. This statistical test allowed us to determine whether the differences between the samples were significant or not. The other procedures are mentioned in the supplementary material.
. Results and discussion
GC/MS analysis of the essential oil
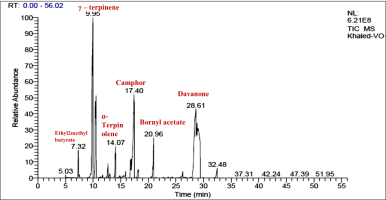
The essential oil that was extracted from the plant material via hydro-distillation was characterized by GC/MS. The chemical composition of the oil is shown in Figure 1 and Table 1. The results showed that the essential oil had a faint blue color and a strong characteristic odor, and was obtained with a yield of 3.2% (v/w).
Table 1
GC MS data of essential oil.
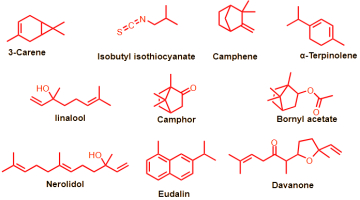
The results of the GC-MS analysis revealed the presence of 25 different compounds, of which 20 constituted 95.1% of the total composition of the oil. These compounds belong to several classes of natural constituents, including oxygenated sesquiterpenes (14.75%), aromatics (7.6%), and esters (6.9%) as well as one ketone (5.21%) and butyl isothiocyanate (4.38%). The main components detected in the sample were as follows: γ-terpinene (20.02%), camphor (15.75%), camphene (9.86%), nerolidol (9.54%), δ-3-carene (8.85%), davanone (5.21%), and isobutyl isothiocyanate (4.38%), as shown in Figure 2. These findings are consistent with the chemical constituents which exhibited varying percentages that were reported by Chhetri et al. (Chhetri et al., n.d.). They found that davanone (49.4%), camphor (29.6%), nerolidol (5.1%), and 4-terpineol (2.0%) were the identified constituents of A. abyssinica oil in three habitats in Yemen, while the chemical composition of A. abyssinica oil in Ethiopia was composed of approximately 67 compounds, accounting for a total of 99.94%.
A high percentage of oxygenated monoterpenes (84%) was found in the essential oil, along with smaller amounts of oxygenated sesquiterpenes (6.45%), monoterpene hydrocarbons (4.41%), and non-terpenic compounds (3.90%). The essential oil had a complex, diverse composition. Another study reported that the oil extracted from different parts of the plant (leaves, stems, and roots) contained 70 different substances, which accounted for 65.94% of the oil components. The highest concentrations of essential oil compounds, including terpinen-4-ol (10.36%), linalool (13.96%), d-limonene (3.15%), and cubenol (5.15%), were found in the foliage. However, only five compounds were isolated in the root’s essential oil, including 1-naphthleneropanol (55.06%), davanone (17.68%), and (E)-nerolidol (7.74%). Regarding the stem’s essential oil, 97.34% was determined to be octadecanoic acid (Asfaw & Demissew, 2015; Tesfay et al., 2022). In conclusion, we found some differences in the composition of chemical constituents in the extracted oil from that reported before, especially the composition and percentages of the identified compounds in other species, which may be due to climate change.
We found a significant discrepancy between our findings and those reported by Guetat et al. (Guetat et al., 2017)
Phytochemical analyses of volatile oils extracted from four Artemisia species native to Saudi Arabia: A. scoparia, A. monosperma, A. sieberi, and A. judaica were conducted. They detected 30 different compounds in the oils, with A. monosperma having the highest oil yield (93.78% of the total). The main component of the essential oil from A. scoparia was sesquiterpenoids hydrocarbons, which made up 83.23% of the total 17 components identified, representing 97.14% of the oil. Butanoic acid (17.87%) was the most abundant compound of all those detected. The other two species, A. judaica and A. sieberi, consisted of 16 and 17 components, which accounted for 87.47% and 94.2% of the total, respectively. Cis-davanone was the most abundant volatile compound in the oil of A. absinthium from Saudi Arabia. The spathulenol content in this species and another one was 30.42% and 28.41%, respectively (Mohammed, 2022).
Biological activity screening
Antimicrobial screening
The antimicrobial activity of the extracted oil was evaluated using a range of bacterial and fungal species. The data are shown in Table 2. The average diameter of the bacteria-growth inhibition zones (IZ) encircling the disks, expressed in millimeters, was used to measure the antimicrobial activity.
The antibacterial activity of the extracted oil was measured by its Minimum Inhibitory Concentration (MIC). The data are shown in Table 3. The oil demonstrated a significant antibacterial effect against some of the bacterial strains tested, such as E. coli, Kl. pneumoniae, St. aureus, St. mutans, and A. ochraceus, as indicated by the inhibition zone diameter and MIC values, which were comparable to those of the positive antibiotic controls: Gentamicin and Ampicillin. However, the oil failed to exhibit any antibacterial activity against P. aeruginosa, A. brasiliensis, and fungi that have developed resistance to antibiotics, which is consistent with previous studies (EL Moussaoui et al., 2021). The results were similar to those reported previously (Kalemba et al., 2002; Ramezani, Behravan & Yazdinezhad, 2005; Zouari et al., 2010), which showed that the oil from A. herba alba grown in Tunisia had high effectiveness against E. coli (11.30 mm), B. cereus (23 mm), and S. aureus (17.70 mm). This antibacterial activity could be due to the presence of borneol, 1,8 cineol, and camphor, which indicates that the oxygenated monoterpenes possess an antibacterial power against various microorganisms (Dorman & Deans, 2000; Faleiro et al., 2003).
Table 2
Diameter of the inhibition zone produced by the essential oil.
Antitumor profile
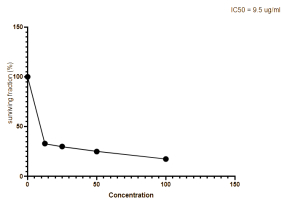
We measured the anti-proliferative activity of the extracted oil on the hepatocellular carcinoma (Hepg2) cell line using the standard MTT assay. We compared the results with those of Adriamycin, which served as a positive control in this experiment. In Figure 3, we summarize these findings by plotting the median growth inhibitory concentration (IC50) values for each drug. The IC50 values represent the amount of isolated oil required to halve cell proliferation after 48 hours of exposure. The oil displayed promising anticancer effects, with an IC50 value of 9.5 μg/ml, while the standard drug Adriamycin had an IC50 value of 6.9 mg/ml. These effects might be due to the presence of certain monoterpenes and davenone ketone in the oil. Akrout et al. reported similar findings (Akrout, Gonzalez, El Jani & Madrid, 2011) and found that the oil of A. campestris growing in southern Tunisia displayed antitumor activity that protected against human colon cancer HT-29 cells. As reported previously (Taleghani, Emami & Tayarani-Najaran, 2020; Romanteeva, Berezutsky & Kurchatova, 2023), this Artemisia species is a promising plant for the treatment of cancer.
Molecular docking profile
Molecular docking is a computer methodology employed to identify ligands or organic compounds that possess both energetic and geometric compatibility with the binding site of a protein. It is employed to examine how multiple molecules interact, such as a protein and its ligand (Azam & Abbasi, 2013). After running the experiment simulations, we undertook further examination by self-docking the isolated oil with the dihydrofolate reductase enzyme from Staphylococcus aureus (PDB: 3SR5) in its original binding site, as determined by X-ray analysis (Li et al., 2011).
Table 4
Binding-affinity energies in (kcal/mol) for monoterpenes, oxygenated monoterpenes, sesquiterpenes, naphthalene, and isothiocyanate against DHFR.
The investigated monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpenes, eudalin, and isothiocyanate exhibited a root mean square deviation (RMSD) lower than 2Å. Following the validated docking procedure for monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpenes, eudalin, and isothiocyanate, we performed redocked simulations at the active site of S. aureus dihydrofolate reductase (PDB: 3SR5).
The current study demonstrated that the constituents of the oil extract could comprehend the amino acid keys of the protein active site by arene-arene, H bond, and arene-cation interactions. The docking scores of DHFR (dihydrofolate reductase) varied, depending on the presence of oxygenated monoterpenes and sesquiterpenes (Table 4, Figure 4 and 5).
Figure 4
Predicted binding mode of oxygenated monoterpenes, sesquiterpenes, eudalin, and isothiocyanate into DHFR; H-bonding is represented in the blue lines, while n-π and π-π bonds are marked with the green line.
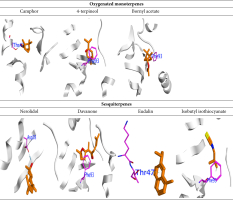
Figure 5
2D binding mode of oxygenated monoterpenes, sesquiterpenes, eudalin, and isothiocyanate into DHFR; H-bonding is represented in the blue lines, while n-π and π-π bonds are marked with the green line.
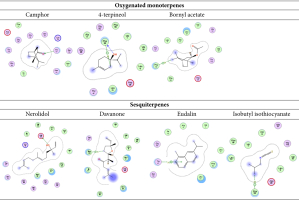
The binding energies and bound structures displayed in Table 4 show that sesquiterpenes have the highest binding affinity, with ΔG = -7.12 and -7.27 Kcal/mol for Davanone and Nerolidol, respectively, and interact with Asp98 and Phe93, while Oxygenated monoterpenes have the highest docking scores (ΔG =-5.07, -5.27, and -5.5 Kcal/mol) for Bornyl acetate, 4-terpineol, and Camphor, respectively, revealing interactions with Thr47 and Phe93, respectively (see Figure 4 and 5). In addition, Isothiocyanate formed a hydrogen bond with Phe93, and the Eudalin compound interacted with Thr47, but the antimicrobial activity of these isolated types is considered to be low. In addition, to explicate the antitumor potency of these desirable oils by analyzing their potential interaction mechanisms with their crystal structures (PDB: 4HJO for the EGFR kinase) (Park, Liu, Lemmon & Radhakrishnan, 2012), the preliminary inhibitors [Erlotinib] were redocked into the EGFR crystal framework to verify the docking methodology. The low RMSD values (0.6 and 1.8 Å, respectively) obtained from the comparison of the original and redocked positions of the co-crystallized inhibitor confirmed the effective performance of the targeted molecules for oil. The binding free energies ΔE are shown in Table 4. The initial inhibitors were adequately installed into their binding sites in order to attain their crystal configurations. The binding pocket showed enhanced stability, with the lowest score and RMSD poses. The docked poses were ranked based on the data, and the most competent docked conformation for each compound was chosen. The hetero ring for Erlotinib and benzimidazole centered on the EGFR adenine-pocket and interacted with ASN54, ASP89, Met 769, Gly772, Asp831, Lys721, and Leu694. The molecule docked prolifically into active sites via a similar mechanism as the original inhibitors.
This study found that the oil components could interact with the protein active site amino acids in different ways, such as H bond, arene-cation, and arene-arene. The results suggested that oxygenated monoterpenes and sesquiterpenes might influence the docking scores with EGFR, as revealed in this study.
Moreover, sesquiterpenes had the highest binding affinity with ΔG = -7.34 and -7.13 Kcal/mol for Davanone and Nerolidol, respectively, and interacted with Thr766 and Thr830. The docking scores for oxygenated monoterpenes indicated their affinity for the receptor. Bornyl acetate, 4-terpineol, and Camphor had ΔG values of -5.27 Kcal/mol, -5.26 Kcal/mol, and -5.6 Kcal/mol, respectively. They interacted with different amino acids at the binding site, such as Asp831, Thr830, Met742, and Phe832. Figure 6 and 7 shows the molecular interactions of these compounds. In addition, Isothiocyanate formed a hydrogen bond with Asp831, and the Eudalin compound interacted with Thr830, but the antimicrobial activity of these isolated types is considered to be low.
Figure 6
Predicted binding mode of oxygenated monoterpenes, sesquiterpenes, eudalin, and isothiocyanate into EGFR; H-bonding is represented in the blue lines, while n-π and π-π bonds are marked with the green line.
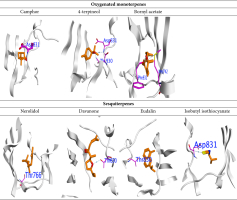
Figure 7
2D binding mode of oxygenated monoterpenes, sesquiterpenes, eudalin, and isothiocyanate into EGFR; H-bonding is represented in the blue lines, while n-π and π-π bonds are marked with the green line.
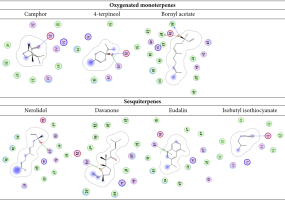
Table 5
Binding-affinity for compounds of oil with the docking score (kcal/mol) against EGFR.
. Conclusion
The Artemisia genus is an essential member of the Compositae family. The chemical constituents of the volatile oil from A. abyssinica, which was extracted via hydro-distillation, were identified using GC/MS analysis. Twenty compounds accounting for 95.1% of the total oil were identified: γ-terpinene, camphor, camphene, nerolidol, δ-3-carene, davanone, and isobutyl isothiocyanate constituents. The essential oil inhibited both Gram-positive and Gram-negative microorganisms to varying degrees. The antitumor potency of the oil was evaluated using the MTT assay, which revealed promising antitumor activity. The molecule docking for antitumor and antimicrobial activity was evaluated based on their potential interaction mechanisms with their crystal structures of DHFR and EGFR kinase.
The interaction of the oil components with protein active sites, through various bonding mechanisms, and their potential effect on DHFR and EGFR docking scores, underscores the therapeutic potential of the oil and warrants additional exploration in pharmacological contexts.