Introduction
Botrytis cinerea Pers.: Fr. and Sclerotinia sclerotiorum (Lib.) de Bary are necrotrophic fungal plant pathogens that affect hundreds of plant species producing gray and white molds, respectively (Boland,1994; Williamson et al.,2007). They infect a wide range of plants via sclerotia, mycelia, or dispersed spores (Clarkson et al.,2003). The sclerotia produce mycelia that penetrate roots and senescent leaves (especially lower leaves), which are in contact with the soil and most susceptible, and then transfer to the stem base and upper leaves, leading to wilt and leaf collapse (Subbarao,1998). Sclerotinia can also attack lettuce at any development stage and any plant part, such as roots, crown, and leaves (Rabeendran et al.,2006). The impact of such mold pathogens varies enormously from year to year, depending on the weather conditions, host plant, and inoculum. For several decades, excessive chemical fungicides have been used to successfully manage the diseases, resulting in increased costs to growers, disrupted natural biological systems, development of pathogen resistance, and adverse effects on other organisms, environment, and human health. Many fungicide groups have been used for mold management (Zhao et al.,2019). Because of their negative effects on human health and environment (Alavanja et al.,2004), integrated pest management (IPM) has been used worldwide as a safety strategy to minimize the risk of pesticides in agriculture (Epstein,2014). IPM is an ecosystem approach to safely protect crop production from the problems produced by chemical pesticides (FAO,2020). Biological control is one such management strategy, as it includes alternative measures (Passera et al.,2017) such as the use of plant extracts (Martinez-Romero et al.,2008) and biological control agents (BCAs) (Camele et al.,2012; Fiume & Fiume,2005) because they considered as safe to humans and the environment. Secondary metabolites used against pathogens can be safely used for biocontrol, as they can be biodegraded to nontoxic components (Camele et al.,2012). Generally, BCAs such as bacteria and fungi can effectively control plant diseases via volatile organic compounds, antibiosis, induction of plant resistance, competition for nutrients and niches, or parasitism. Most bacterial BCAs such as Bacillus subtilis have achieved their high potential to control Botrytis cinerea with antibiosis as their major mode of action (Calvo-Garrido et al.,2019). Some microbial protectants derived from Bacillus subtilis induce systemic acquired resistance (SAR) that controls Botrytis and Sclerotinia rots, for example, Sonata (AgraQuest, Davis, CA, USA; https://www.bayercropscience.us/products/fungicides/sonata) and Serenade (AgraQuest; https://www.cropscience.bayer.us/products/fungicides/serenade). Plant extracts are substances extracted from different plant parts; they contain many compounds involved in antimicrobial activity and have been classified in groups, mainly as essential oils or phenolic compounds (Fiume & Fiume,2005). Cinnamon, Cinnamomum zeylanicum J. Presl., contains eugenol [only 8% volatile oil and 75% cinnamic aldehyde (3-phenyl-2-propenal)], whereas moringa (Moringa oleifera) leaves and seed extracts contain fatty acids, glycosides, proteins, alkaloids, tannins, niazirin, terpenoids, flavonoids, and phenolic compounds, which are thought to be responsible for their antimicrobial activities. Such natural compounds could be used to increase the income of farmers by decreasing the crop yield losses caused by fungal molds. In the current study, we aimed to develop alternative natural compounds for biocontrol assessment by examining the inhibition efficacy of different sources of potential biofungicides, such as some plant extracts, BCAs, and organic compounds, against the most important fungal molds.
Material and Methods
Fungal Isolates and Plant Materials
Five isolates of Botrytis cinerea (BF, BCL, BCS, BCC, and BCP) and five isolates of Sclerotinia sclerotiorum (SSP, SSM, SSPo, SSTo, and SSB) were collected from different locations and host plants (Table 1), cultured on potato dextrose agar (PDA; Difco Laboratories, USA), and incubated at 23 °C for 7 days. Their virulence levels were tested on both lettuce and broad bean leaves. Isolates with the highest virulence level on lettuce leaves were selected for the subsequent biocontrol assays both in vitro and in vivo.
Table 1
Collection of Botrytis and Sclerotinia isolates.
Pathogenicity Assay
Mycelial plugs were cut from the colony margin of each 7-day-old fungal culture (diameter 6 mm) by using a sterilized cork-borer and transferred to detached leaves of sensitive domestic cultivars of lettuce (Lactuca sativa L. ‘Baladi’) and broad bean (Vicia faba L. ‘Misr 3’). For each isolate, four replicates were prepared, placed on moist towels in plastic boxes covered to maintain high humidity, and incubated at 23 °C. The isolate virulence was assessed by measuring the lesion diameters after 3 days of incubation at 23 °C. This pathogenicity assay was repeated twice.
Preparation of Biotreatments
The potential biocontrol agents Trichoderma harzianum (TH) and Bacillus subtilis (BS) (Department of Plant Pathology, Faculty of Agriculture, Ain Shams University, Egypt) were prepared and maintained on PDA at 4 °C for further analysis. Different plant extracts and organic compounds were prepared from Moringa oleifera (Mor), Cinnamomum zeylanicum (Cin), Cactus spp. (Agr), and amino acid derivatives (Aad) at 5%, 0.1%, 0.2%, and 0.4%, respectively, according to our preliminary study (data not shown). Teldor (Tel) at 0.05% was used as the positive control. The negative controls were not subjected to any treatment (Con-) prior to the in vitro and in vivo tests.
Dual Culture Test and Mycelial Growth Measurement In Vitro
Each prepared concentration of the potential biofungicides was mixed with 15 mL of PDA and inoculated with each of the previously mentioned isolates. The two BCAs, TH and BS, were first assessed as growth antagonists by pairing them with highly virulent isolates on PDA plates. Growth plugs (0.6 cm in diameter) from the colony edge of each potential BCA and fungal pathogen were placed on opposite sides of the PDA plates. Botrytis and Sclerotinia isolates were also inoculated separately on PDA plates to serve as controls. Three plates were prepared for each pathogen isolate cultured on PDA amended with the plant extract, fungicide, or potential BCA combinations and incubated at 23 °C for 3 days. Mycelial growth (MG) of each pathogen isolate was measured daily for 3 days after the control plates were fully covered with mycelia.
Biocontrol Assay In Vivo
To investigate the inhibitory effect of the biotreatments described above on lesion formation, healthy detached leaves of lettuce and broad bean were prepared. Treatments were administered using either one of the two bioagents, BS and TH, or the natural compounds Cin, Agr, Mor, and Aad, which were prepared at adjusted concentrations of 0.1%, 0.2%, 5%, and 0.4%, respectively, based on our preliminary study (data not shown). Each experiment consisted of four replicates of detached leaves precoated with each biotreatment, and distilled water (Con-) and the commercial fungicide (Tel) were used as the negative and positive controls, respectively. The precoated leaves were left on laboratory benches for a day, to let the surface of the detached leaves dry, and subsequently inoculated with the pathogen. Each leaf was divided into two parts along the vein, the left part was inoculated with a PDA plug as the negative control, and the right part was inoculated with the pathogen isolate. The spore concentration of TH was adjusted to 108 spores/mL by using a hemocytometer. An appropriate concentration of BS was also prepared at 5 × 107 CFU mL−1. The isolates with the highest virulence level were inoculated on either lettuce or broad bean leaves. The inoculated leaves were maintained in a covered moist chamber at room temperature. The lesion diameter was assessed after 3 days of inoculation. The efficacy of each treatment was calculated according to Abbott’s formula (Abbott,1987):
Statistical Analysis
All data were statistically analyzed (SAS,2006) using one-way analysis of variance. The mean values of each independent experiment (means ± SE) were obtained to compare the differences among the treatments by using the Duncan test at p < 0.05.
DNA Extraction and Real-Time Quantitative PCR (qPCR)
DNA extraction was performed from some plant samples that were treated with Tel or untreated and inoculated with one of the most virulent isolate to check for its presence within the asymptomatic samples. The genomic DNA extraction kit of QIAGEN (DNeasy Plant Mini Kit; cat No. 69104) was used for the extraction, and PCR was conducted using the specific primer pair Bc-f (5′-CAGGAAACACTTTTGGGGATA-3′) and Bc-r (5′-GAGGGACAAGAAAATCGACTAA-3′) designed by Fan et al. (2015). The total PCR volume was 20 μL and consisted of 5 μL of genomic DNA (20 ng/μL), 1 μL of each primer (Bio-search Technologies; 600 nM), 10 μL of the SYBR Green master mix “Bio-line,” and 3 μL of H2O. The amplification program was performed using the QIAGEN system [Rotor-Gene Q Series Software 2.0.3 (Build 2)] as follows: initial denaturation at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 10 s, annealing at 51 °C for 30 s, and extension at 72 °C for 45 s. For the standard curve, a serial dilution of the positive control was prepared from the extracted DNA of B. cinerea. The negative controls (NTC – no template control; HPC – a healthy plant control) were included in each run. This amplification test was performed twice.
DNA Quantification
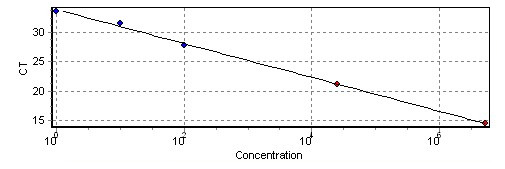
The DNA quantification was performed by plotting unknown sample concentrations on the standard curve of a serial dilution of known concentration. The standard curve was established using a plot of the cycle threshold (Ct) against the logarithm of the known amount of the pathogen’s DNA. A linear regression analysis of the standard plot was used to calculate the amount of pathogen DNA in the tested samples.
Results
Virulence Diversity of Botrytis and Sclerotinia Isolates by Using Detached Lettuce and Broad Bean Leaves
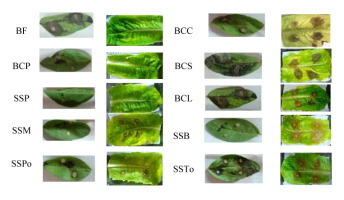
The pathogenicity test revealed a symptom divergence after 3 days of inoculation, depending on the isolate and plant species. The dimensions of the infection lesion around the culture plug of each isolate differed among the lettuce and broad bean leaves because of the virulence level. Apart from the negative control (PDA plug), two distinct symptoms were observed on the leaves, a low and high virulence level. Generally, the degree of infection by the BCP, SSPo, and SSM isolates on the lettuce and/or broad bean leaves was low and ranged from 0.2 to 0.5 cm (Figure 1), whereas that by the BCS, BCL, BCC, and SSTo isolates on both lettuce and broad bean leaves was high and >0.5 cm (Figure 1). Moreover, the BF and SSB isolates did not cause any symptoms on lettuce and broad bean leaves, respectively, but caused a high infection on broad bean and lettuce leaves, respectively (Figure 1). In addition, the SSP isolate caused no symptoms on both lettuce and broad bean leaves (Figure 1). The most virulent isolates tested on the detached lettuce leaves were selected for the subsequent biocontrol analysis.
Effects of Different Biomeasures on the Mycelial Growth of BCS and SSTo Isolates In Vitro
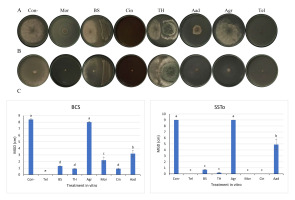
One-way SAS was performed to evaluate significant differences (p < 0.05) across mycelial growth diameters of BCS and SSTo isolates, which demonstrated the highest virulence on lettuce and broad bean leaves (Figure 1). The positive control Tel exhibited the highest inhibition (100%) against BCS, followed by Cin, TH, BS, Mor, and Aad (89%, 89%, 85%, 75%, and 62%, respectively); the inhibition value of Agr was insignificant (Figure 2A,C). In addition, the highest inhibition of SSTo growth was 100% using Tel, Mor, and Cin; TH and BS, 98% and 92%, respectively, and Aad, 46% (Figure 2B,C). TH, BS, Cin, and Mor treatments most effectively inhibited mycelial growth of the BCS and SSTo isolates, whereas Aad treatment caused the lowest mycelial growth suppression in both isolates in vitro (Figure 2C).
Figure 2
Mycelial growth inhibition assay. (A) BCS and (B) SSTo isolates grown on PDA subjected to various treatments after 3 days of incubation at 23 °C. (C) Graphs show significant differences among mycelial growth dimensions (MGD) of BCS and SSTo isolates following different treatments, as per Duncan’s test (p < 0.05).
Effects of Different Biomeasures on Botrytis cinerea and Sclerotinia sclerotiorum In Vivo
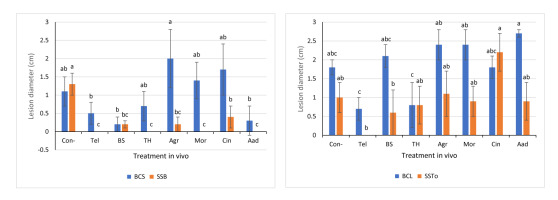
Significant differences (p < 0.05) were observed across the infection degrees of BCL, BCS, SSB, and SSTo isolates (Figure 3). Duncan’s multiple range test was used to identify treatments that significantly differed under different experimental conditions. However, our objective in using SAS was to not test for differences within each experiment but eliminate treatments that provided no useful information to discriminate among different biomeasures. The infection severity of BCS, BCL, SSB, and SSTo isolates, which demonstrated high virulence levels on lettuce leaves (Figure 1), was statistically analyzed. The highest inhibitory effects against infection caused by the BCS isolate were observed with Aad and BS treatments (73% and 82%, respectively), when compared with the positive control (Tel, 64%); the other treatments did not show any significant inhibitory effects (Figure 3A). Moreover, the inhibition efficacy was 100% against SSB infection with TH, Mor, and Aad treatments as well as treatment with the positive control (Tel, 100%). These treatments significantly differed from BS, Agr, and Cin treatments, which also showed high efficacy against SSB (86%, 86%, and 71%, respectively; Figure 3A). In addition, a significantly high inhibition of infection was observed against the BCL isolate with Tel and TH treatments (61% and 56%, respectively), in comparison with other treatments that did not show any inhibitory effects (Figure 3B). Tel treatment also showed the highest inhibitory effect against the SSTo isolate (100%), followed by BS treatment (39%). However, no inhibitory effects against the SSTo isolate were observed with the other treatments (Figure 3B).
Effects of Biotreatments Against Different Infection Backgrounds
The efficacy of the currently used biotreatments was also tested using either two different host plants (lettuce and broad bean leaves) or different aggressive isolates, in order to demonstrate their context-dependent effects. In some inoculated leaves, infection lesions with a water-soaked, brownish to dark brownish color and soft wet rot were observed. Some leaves appeared damaged and were therefore excluded during the measurement. Infection lesions produced by the BCS isolate were observed on both lettuce and broad bean leaves (Figure 4A,B). The highest efficacy of inhibition against the BCS isolate was observed on both lettuce and broad bean leaves following TH, Tel, and BS treatments (65%, 75%, and 85%, respectively; Figure 3A, Figure 4A,B). In addition, Aad treatment was effective against only the BCS isolate on lettuce leaves (Figure 3A, Figure 4A), whereas Agr, Mor, and Cin treatments did not inhibit the BCS isolate on both lettuce (Figure 3A, Figure 4A) and broad bean (Figure 4B) leaves. Furthermore, the highest efficacy of inhibition against the BCC isolate was observed following Tel, BS, Agr, Mor, Aad, and Cin treatments (87%, 73%, 73%, 80%, 80%, and 53%, respectively), followed by TH treatment (Figure 4C). Interestingly, the maximum inhibitory effect was 100% against the SSB isolate with Tel, Mor, TH, and Aad treatments, as shown previously (Figure 3A, Figure 4D), followed by BS, Agr, and Cin treatments, which also demonstrated high inhibitory effects (Figure 3A, Figure 4D). Generally, the bioagents BS and TH showed the highest inhibitory effects against the BCS isolate on both lettuce and broad bean leaves (Figure 3A, Figure 4A,B), and then against BCL (Figure 3B), BCC (Figure 4C), and SSB isolates (Figure 3A, Figure 4D). Moreover, Aad treatment showed high inhibitory effects against BCS (Figure 3A, Figure 4A), BCC (Figure 4C), and SSB (Figure 4D) isolates on lettuce leaves, whereas Mor treatment inhibited only BCC and SSB isolates.
Molecular Detection of Botrytis in Lettuce Leaves
The results of qPCR conducted using lettuce samples (Con- and Tel) yielded positive amplification curves that corresponded to the presence of Botrytis in the tested samples in different qPCR cycles (Figure 5). The results showed pathogen quantities of 5.3 mg/μL and 25.3 μg/μL corresponded to the samples Con- and Tel, respectively (Table 2), by using the standard curve that demonstrated an amplification efficiency reached 97% (Figure 6). This observation indicated the presence of either obvious infection or latency of Botrytis (Table 2).
Figure 5
qPCR amplification of Botrytis cinerea in some tested lettuce leaves. Sample colors: see Table 2.
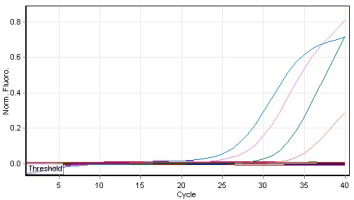
Figure 6
Standard curve revealed a linear relationship (R2 = 0.97) between the DNA concentration of serial dilutions of target genomic DNA of Botrytis cinerea and the cycle threshold (CT).
Discussion
Virulence divergence of Botrytis and Sclerotinia was evident depending on the isolate and host plant, lettuce and broad bean. Because of the close relationship between the two pathogens, different characteristics seemed comparable. Infection degree was determined depending on isolate as reported previously (Aboelghar et al.,2019); except the BF isolate (Botrytis fabae), which is specific to broad bean infection. To biocontrol the fungal molds Botrytis and Sclerotinia, many biomeasures such as plant extracts, organic compounds, and BCAs were tested, as they are considered important for plant disease control (Abdel Wahab & Helal,2013; SIBAT,). The current study was conducted to test many biomeasures such as plant extracts (Mor and Cin), organic compounds (Aad and Agr), and BCAs (BS and TH) either in vitro or in vivo to test their effectiveness against highly virulent isolates of Botrytis and Sclerotinia. The results demonstrated that Mor, Cin, BS, and TH were the most effective inhibitors of both fungal molds in vitro and in vivo. In addition, the absence of mycelial growth in SSTo was notable with Mor and Cin treatments, which caused 100% inhibition in vitro, indicating their effectiveness as chemical fungicides against the fungal molds. In fact, the potential antifungal activity of Moringa oleifera leaves is attributable to many antimicrobial compounds such as fatty acids, alkaloids, proteins, niazirin, and glycosides (Sibat,20). Cinnamomum zeylanicum contains eugenol (4-allyl-2- methoxyphenol), which is a naturally occurring phenolic compound and may disrupt the cellular membrane, leading to cell death (Chunmei et al.,21). Furthermore, the results showed that the two BCAs, BS and TH, exhibited a significant antifungal activity (85–100%) against the fungal molds in vitro and in vivo, depended on isolate and host plant. Antagonists can inhibit pathogens by antibiosis, some bioactive metabolites, and active degradation-related enzymes, which play crucial roles in inhibiting Botrytis cinerea and Sclerotinia sclerotiorum (Elad,22,23). Other modes of action have also been investigated on Pythium oligandrum that could protect plants against many fungal pathogens by secreting elicitors that trigger plant defense (Brozová,24; Le Floch et al.,25). The highest inhibitory effect (100%) was achieved against some isolates with Mor, Aad, and TH treatments, which caused a significantly higher reduction in both fungal mycelial growth and infection lesion diameter, than the positive control, Tel. The results showed that TH, BS, Cin, Mor, and Aad treatments inhibited either mycelial growth or infection lesion, depending on the isolate. Aad is an amino acid derivative compound that effectively inhibited BCS, SSTo, SSB, and BCC isolates either in vitro or in vivo. In fact, increasing plant tolerance to many stress conditions by the accumulation of some amino acid derivatives intracellularly has been reported previously (Bonaterra et al.,26), confirming their inhibitory effects. In addition, the plant extract Cin appeared to be the most effective inhibitor of the tested isolates in vitro, whereas the plant extract Mor was an effective inhibitor of BCS and SSTo isolates in vitro or BCC and SSB isolates in vivo. Moreover, the inhibitory efficacy of the biotreatments Agr, Tel, BS, Mor, Aad, and Cin was 53–87% against the infection produced by the BCC isolate on lettuce leaves. Primarily, the inhibitory efficacy of the BCAs, Cin, Mor, and Aad was significant when the leaves were treated 24 hr prior to pathogen inoculation; thus, they play a biocontrol role to prevent fungal molds. Such effective inhibition could be due to the bioactive components produced by the plant extracts/bioagents and may play an important role in the inhibitory effect on the fungal molds. Even though the biomeasures Cin and Mor demonstrated a positive effect in the in vitro tests, they did not exhibit a similar effect on some isolates in the in vivo study. This could be due to their tested concentrations, which did not appear to be sufficient for the in vivo experiments. Biopesticides have different modes of action and are safe for humans and the environment; they are the best choice to control plant diseases and avoid fungal resistance, which is increasingly observed in different fungicide groups such as carbendazim, diethofencarb, iprodione, and pyrimethanil (Zhao et al.,6). Thus, biological control is undoubtedly the best choice to manage important crop diseases (Fiume & Fiume,13; Haidar et al.,27; Marín et al.,28; Nicot et al.,29; Passera et al.,10; Sylla et al.,30). Further experiments should be performed to evaluate the current efficient biomeasures for other host plants, as they could be potential biofungicides that may be used for reducing the damage caused by fungal mold diseases.
Handling Editor
Ewa Zalewska; University of Life Sciences in Lublin, Poland; https://orcid.org/0000-0001-7445-9808